Understanding Heating And Cooling Curves Worksheet
Understanding Heating And Cooling Curves Worksheet Heating&cooling curves a)decreases b)increases c)remains the same 39.as a liquid boils at its normal boiling point, its temperature base your answers to questions 40 through 42 on the information below. starting as a gas at 206°c, a sample of a substance is allowed to cool for 16 minutes. this process is represented by the cooling curve below. Language: english (en) id: 1365575. 07 09 2021. country code: bb. country: barbados. school subject: chemistry (1061818) main content: states of matter (2073709) from worksheet author: this worksheet is seeks to link the changes of state of matter and how particles move when heat is added or removed and represented as a graph.

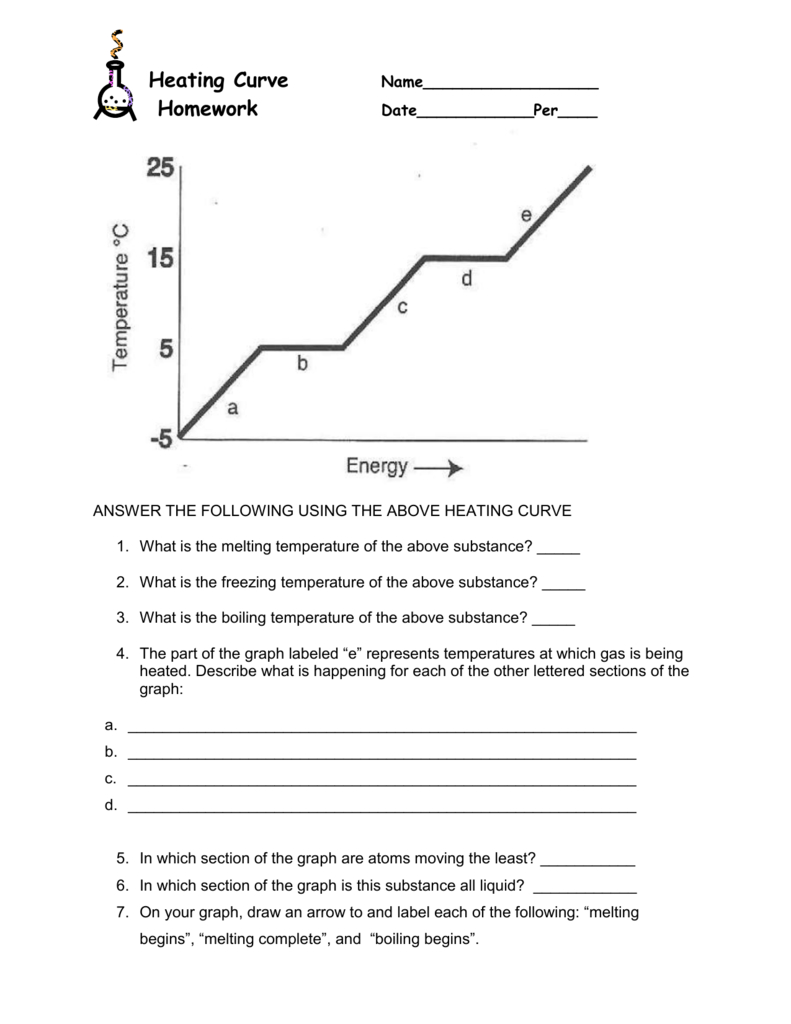
Heating And Cooling Curves Worksheets Heating curve of substance x 20 22 24 26 28 30 80 75 70 60 55 temp. (oc) 5 0 40 35 30 25 20 15 10 12 14 16 time (minutes) 18 the heating curve shown above is a plot of temperature vs time. Thermodynamics unit – specific heat and heating curves 1. what is the difference between a calorie and a joule? (which is bigger, by how much?) 1 cal = 4.184 j a calorie is bigger than a joule by more than 4 times. 2. heat is exchanged from warmer substances. With 10 questions on each worksheet, students will apply their knowledge of heating and cooling curves, phase changes, and energy transfer. these worksheets can be utilized as in class activities, homework assignments, or even as part of exam preparation. engage your students with this valuable heating and cooling curve worksheet bundle. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down.

Comments are closed.