Tungsten Uses Properties Facts Britannica

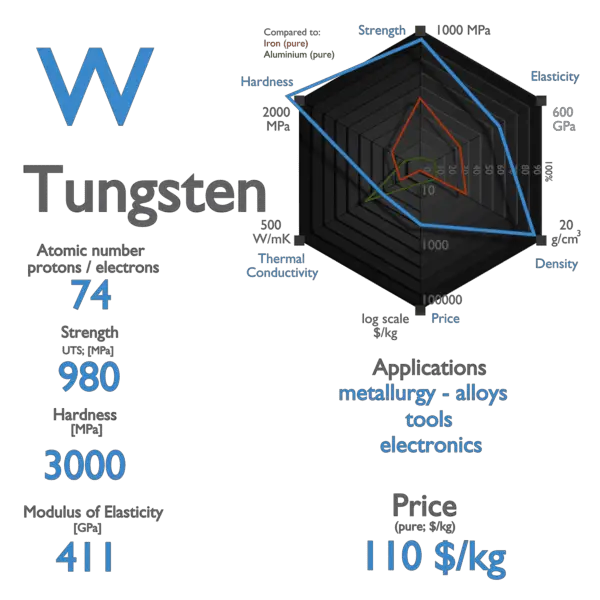
Tungsten Uses Properties Facts Britannica Tungsten metal has a nickel white to grayish lustre. among metals it has the highest melting point, at 3,410 °c (6,170 °f), the highest tensile strength at temperatures of more than 1,650 °c (3,002 °f), and the lowest coefficient of linear thermal expansion (4.43 × 10 −6 per °c at 20 °c [68 °f]). tungsten is ordinarily brittle at room. Melting point. 6,152 °f (3,410 °c) specific gravity. 19.3. the amount of tungsten in earth’s crust is estimated to be 1.5 parts per million, or about 0.05 ounce (1.5 grams) per ton of rock. tungsten is about as abundant as molybdenum, which it resembles, and tin. tungsten is about half as plentiful as uranium.

Tungsten Uses Properties Facts Britannica Tungsten carbide is immensely hard and is very important to the metal working, mining and petroleum industries. it is made by mixing tungsten powder and carbon powder and heating to 2200°c. it makes excellent cutting and drilling tools, including a new ‘painless’ dental drill which spins at ultra high speeds. Some other tungsten alloys and their uses are mentioned below: 3. aerospace and automotives. tungsten alloys are used in aerospace and automotive industries for the purposes such as manufacturing rocket engine nozzles, turbine blades, etc. it is used for making alloys like high speed steel, hastelloy, stellite, etc. Introduction. tungsten is a chemical element identified by the symbol "w" and the atomic number 74. this element is known for its exceptional strength, high melting point, and usage in a variety of applications, ranging from light bulb filaments to aerospace technology. tungsten is a transition metal characterized by its grayish white. Is a block d, group 6, period 6 element. the number of electrons in each of tungsten's shells is 2, 8, 18, 32, 12, 2 and its electron configuration is [xe] 4f the tungsten atom has a radius of 137.pm and its van der waals radius is 200.pm. in its elemental form, cas 7440 33 7, tungsten has a grayish white, lustrous appearance.

Tungsten Uses Properties Facts Britannica 57 Off Introduction. tungsten is a chemical element identified by the symbol "w" and the atomic number 74. this element is known for its exceptional strength, high melting point, and usage in a variety of applications, ranging from light bulb filaments to aerospace technology. tungsten is a transition metal characterized by its grayish white. Is a block d, group 6, period 6 element. the number of electrons in each of tungsten's shells is 2, 8, 18, 32, 12, 2 and its electron configuration is [xe] 4f the tungsten atom has a radius of 137.pm and its van der waals radius is 200.pm. in its elemental form, cas 7440 33 7, tungsten has a grayish white, lustrous appearance. Physical characteristics. tungsten is a transition metal. raw tungsten steel grey metal that is hard and brittle. it has very high melting point about, 3422 o c and boiling point is about 5930 o c. it has ability to retain its strength at very high temperatures. its density is also very high, about 19.3 times that of water. Tungsten in practice. industrial uses and applications. tungsten is known for its exceptional properties, making it highly valued in various industries. one notable application of tungsten is in the manufacturing of filaments for light bulbs and electronic devices, due to its high melting point and good electrical conductivity 1.

Tungsten Uses Properties Facts Britannica Physical characteristics. tungsten is a transition metal. raw tungsten steel grey metal that is hard and brittle. it has very high melting point about, 3422 o c and boiling point is about 5930 o c. it has ability to retain its strength at very high temperatures. its density is also very high, about 19.3 times that of water. Tungsten in practice. industrial uses and applications. tungsten is known for its exceptional properties, making it highly valued in various industries. one notable application of tungsten is in the manufacturing of filaments for light bulbs and electronic devices, due to its high melting point and good electrical conductivity 1.
Tungsten Uses Properties Facts Britannica 57 Off

Comments are closed.