The Future Of Non Muscular Invasive Bladder Cancer Management

Pdf Cua Guidelines On The Management Of Non Muscle Invasive Bladder Non muscle invasive bladder cancer is a diverse subclassification of urothelial bladder cancer with a wide variation in recurrence and progression rates. risk adapted treatment is essential to balance adverse effects of treatment with adequate disease control and there are many nomograms and strategies to assist, each with specific limitations. The management of non muscle invasive bladder cancer (nmibc) has evolved from the first reports on bladder endoscopy and transurethral resection to the introduction of adjuvant intravesical treatment. however, disease recurrence and progression remain an ongoing risk, placing a heavy burden on healthcare resources and on patients' quality of life.
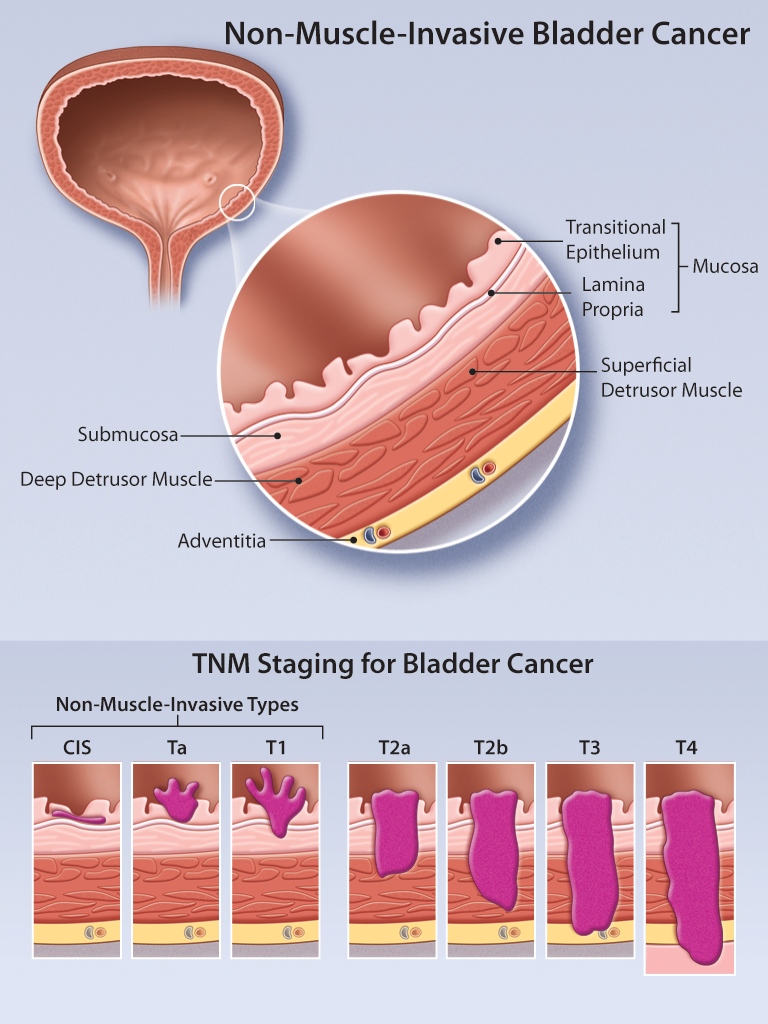
Non Muscle Invasive Bladder Cancer Review Of Diagnosis And Manageme Guideline statement 1. at the time of resection of suspected bladder cancer, a clinician should perform a thorough cystoscopic examination of a patient’s entire urethra and bladder that evaluates and documents tumor size, location, configuration, number, and mucosal abnormalities. (clinical principle) discussion. The landscape of treatment for non muscle invasive bladder cancer is rapidly changing. a complete and careful transurethral resection is the mainstay of initial treatment and is followed by intravesical therapy in intermediate or high risk cases. the standard of care is intravesical bcg. many alternative or additive approaches to this are being. 5. the most important prognostic factors for recurrence and progression of non muscle invasive bladder cancer (nmibc) are stage and grade (le 2). all patients with bladder cancer should be properly staged and, specifically for nmibc, reporting grade is paramount for further management decisions (le 2, strong recommendation). 6. The clinical trial planning meeting for non muscle invasive bladder cancer is a unique opportunity provided by the national cancer institute to bring together leaders in the field including cooperative group investigators, academia, industry, patient advocacy, and officials from fda and nci to spur the development of state of the art trials in non muscle invasive bladder cancer.

Comments are closed.