Standard Electrode Potentials Chemguide At Richard Reddish Blog
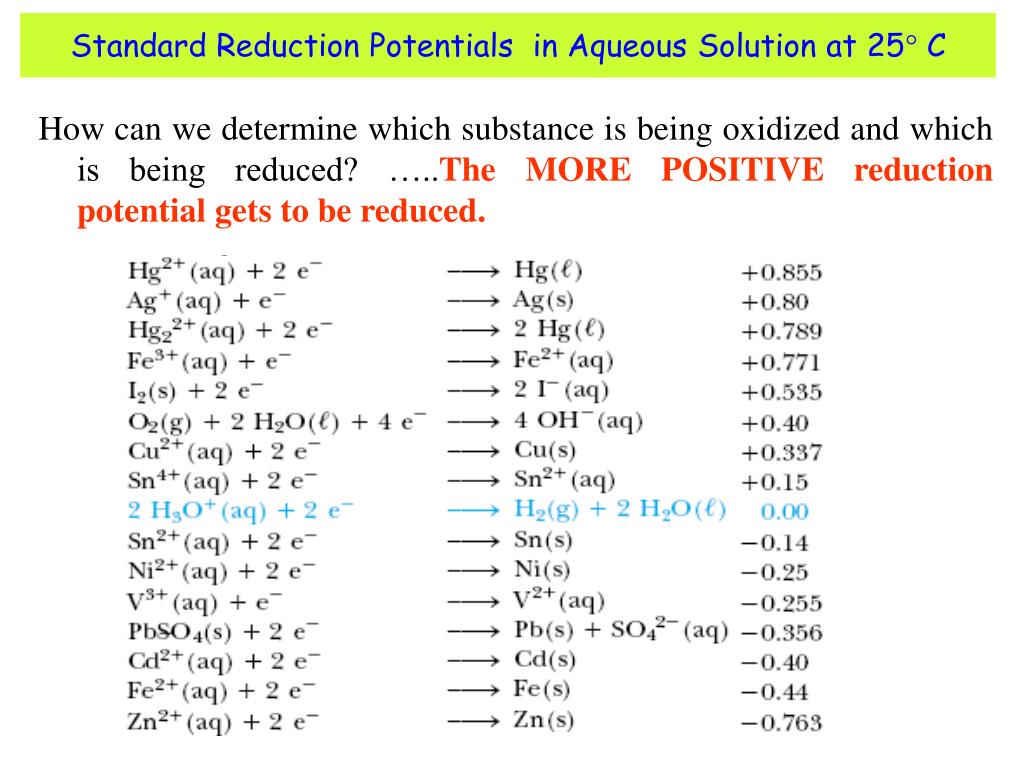
Standard Electrode Potentials Chemguide At Richard Reddish Blog The values that we have just quoted for the two cells are actually the standard electrode potentials of the mg 2 mg and cu 2 cu systems. the emf measured when a metal metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal metal ion combination. A reminder of what you need to know. standard electrode potentials (redox potentials) are one way of measuring how easily a substance loses electrons. in particular, they give a measure of relative positions of equilibrium in reactions such as: the more negative the e° value, the further the position of equilibrium lies to the left.

Standard Electrode Potentials Chemguide At Richard Reddish Blog Don't forget to learn the conditions relating to the word "standard" from further up that page. statement 24.2.2. this is about the standard hydrogen electrode. you will find that on the same page. statement 24.2.3. you will also find the measurement of the standard electrode potentials of metals in contact with their ions on the same page. 24.2: standard electrode potentials. learning outcome 24.2.7. this statement asks that you should be able to work out equations for redox equations from electron half equations. you may well be able to do this already. read the page about writing ionic equations for redox reactions. you can ignore the link at the bottom of the page to a further. The test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. you can find out this relative tendency by looking at the e° values. that means that any redox reaction could be discussed in a similar way. the rest of the examples on this page illustrate this. 24.2: standard electrode potentials. learning outcome 24.2.6. this statement looks at how electrode potentials tell you about oxidising and reducing ability. read the statement before you go on. now is the time to re read three pages about e° values and oxidising and reducing ability. start with the page the electrochemical series.
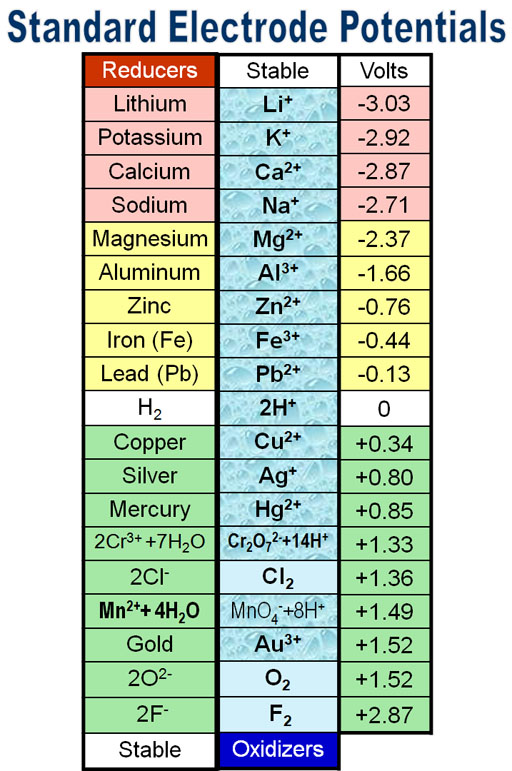
Standard Electrode Potentials Chemguide At Richard Reddish Blog The test tube reaction happens because of the relative tendency of the zinc and copper to lose electrons to form ions. you can find out this relative tendency by looking at the e° values. that means that any redox reaction could be discussed in a similar way. the rest of the examples on this page illustrate this. 24.2: standard electrode potentials. learning outcome 24.2.6. this statement looks at how electrode potentials tell you about oxidising and reducing ability. read the statement before you go on. now is the time to re read three pages about e° values and oxidising and reducing ability. start with the page the electrochemical series. Standard electrode potential. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. the iupac "gold book" defines it as; "the value of the standard emf (electromotive force) of a cell in which molecular hydrogen under standard pressure is oxidized to solvated protons at the left. The potential of a half reaction measured against the she under standard conditions is called the standard electrode potential for that half reaction.in this example, the standard reduction potential for zn 2 (aq) 2e − → zn(s) is −0.76 v, which means that the standard electrode potential for the reaction that occurs at the anode, the.
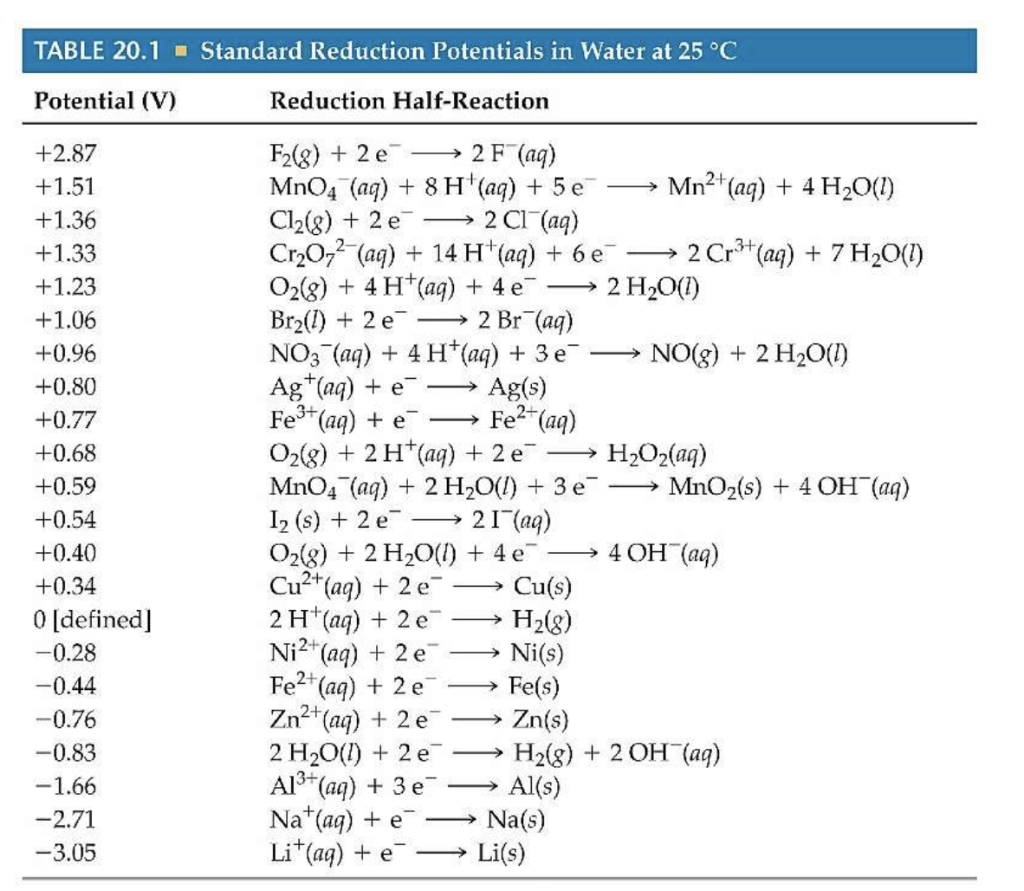
Standard Electrode Potentials Chemguide At Richard Reddish Blog Standard electrode potential. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. the iupac "gold book" defines it as; "the value of the standard emf (electromotive force) of a cell in which molecular hydrogen under standard pressure is oxidized to solvated protons at the left. The potential of a half reaction measured against the she under standard conditions is called the standard electrode potential for that half reaction.in this example, the standard reduction potential for zn 2 (aq) 2e − → zn(s) is −0.76 v, which means that the standard electrode potential for the reaction that occurs at the anode, the.

Comments are closed.