Solved Fill In The Name And Empirical Formula Of Each Ionicо

Solved Fill In The Name And Empirical Formula Of Each Ionicођ There are 3 steps to solve this one. solution. answered by. chemistry expert. step 1. solution for part (a): fe a 2 and clo a 3 a −: empirical formula: view the full answer step 2. The empirical formula of an ionic compound represents the simplest ratio of the ions involved. here are the names and empirical formulas of the ionic compounds that could be formed from the ions in your table:.
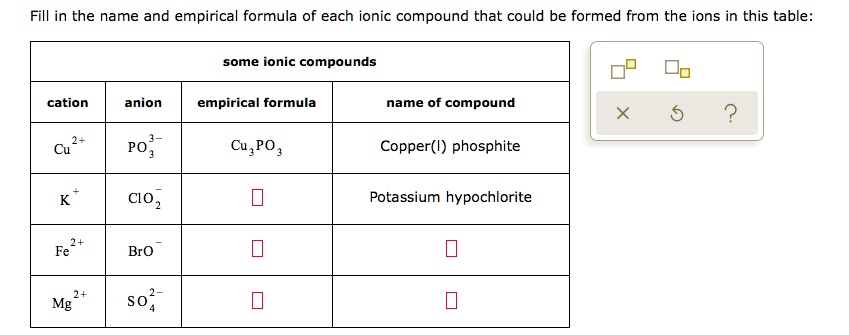
Solved Fill In The Name And Empirical Formula Of Each Ionicођ Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table na i ,na cl ,na br ,na f. Here are the names and empirical formulas of the ionic compounds that could be formed from the given ions: pb2 and cn the compound formed from these ions is lead(ii) cyanide. the empirical formula is pb(cn)2. the subscript '2' is used to balance the charges of the ions. mg2 and co32 the compound formed from these ions is magnesium carbonate. Let's first set up the stable here: cat iron and iron empirical formula and the name of the compound. so first we have p b, 2 plus and s o 42 minus. so this would give me empirical formula here of p b s o 4, and the name here will be led led is multis roman numeral 2 and s o 42 minus…. Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: some ionic compounds cation anion empirical formula name of compound mn? noz mn (no2 , manganese (ii) nitrite 2 zn 2 oh zn (oh) 2 zinc hydroxide mg2 so mgs 02 magnesium sulfite.
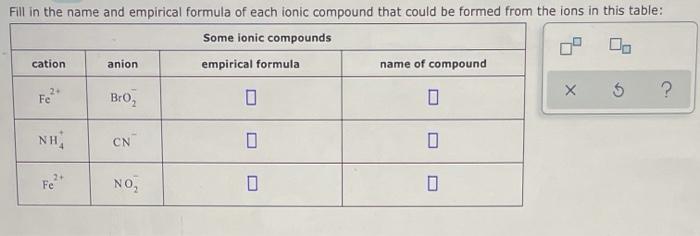
Solved Fill In The Name And Empirical Formula Of Each Ionicођ Let's first set up the stable here: cat iron and iron empirical formula and the name of the compound. so first we have p b, 2 plus and s o 42 minus. so this would give me empirical formula here of p b s o 4, and the name here will be led led is multis roman numeral 2 and s o 42 minus…. Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: some ionic compounds cation anion empirical formula name of compound mn? noz mn (no2 , manganese (ii) nitrite 2 zn 2 oh zn (oh) 2 zinc hydroxide mg2 so mgs 02 magnesium sulfite. Strontium fluoride is an ionic compound , with formula sr f2 . let us see how we got the answer; look at the electronic arrangement of sr and f atom. it loses two electron in its 5s subshell to achieve stability and forms ion sr2. View the full answer step 2. unlock. answer. unlock. previous question next question. transcribed image text: fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: \begin {tabular} {|c|c|c|c|} \hline \multicolumn {3} {|c|} { some ionic compounds } \\ \hline cation & anion & empirical.

Comments are closed.