Sis Certifications On Twitter The Iso 13485 2016 Standard Specifies

Sis Certifications On Twitter The Iso 13485 2016 Standard “the iso 13485:2016 standard specifies requirements for the medical device development & quality management system that should be used by medical device related. Iso 13485 certification involves building a quality management system for medical devices by identifying potential risks and documenting them effectively. the threats may arise from contamination of equipment or errors during handling. iso 13485 provides for analyzing those threats and planning appropriate actions to prevent those risks.
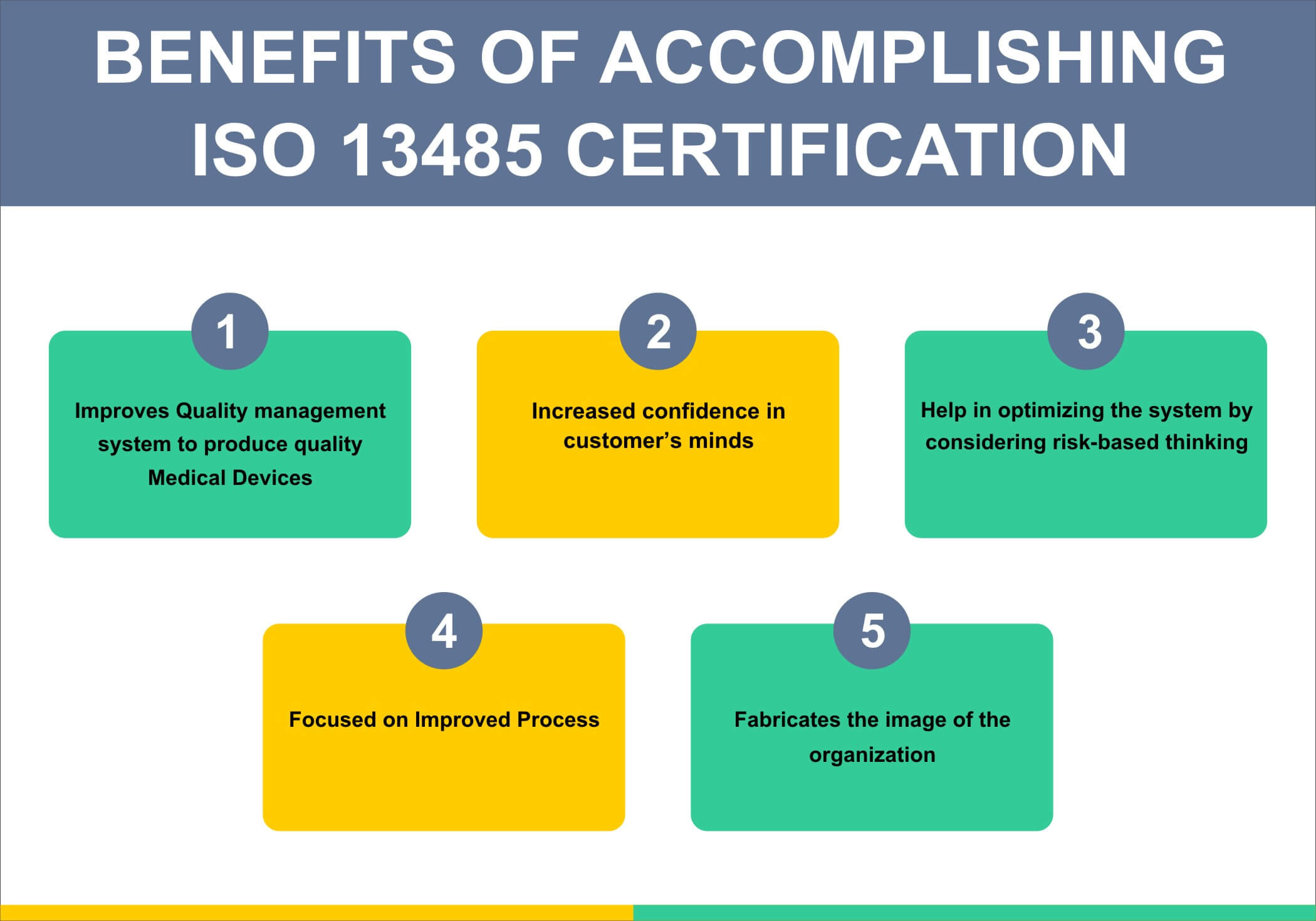
Iso 13485 Certification Process Of Iso 13485 Certification Sis Cert Iso 13485:2016 the gold standard in medical device quality management for organizations involved in the life cycle of medical devices, compliance with iso 13485 is paramount. it's more than a. Iso 13485 quality management system. the iso 13485:2016 is a useful standard because it specifies requirements for a quality management system (qms) when an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Iso 13485 is designed to be used by organizations involved in the design, production, installation and servicing of medical devices and related services. it can also be used by internal and external parties, such as certification bodies, to help them with their auditing processes. An iso 13485:2016 audit is a comprehensive evaluation process conducted to verify that a medical device manufacturer’s quality management system (qms) meets the requirements of the iso 13485:2016 standard. this standard specifies the requirements for a qms that demonstrate the ability to consistently meet customer and regulatory requirements.

Sis Certifications On Twitter The Iso 13485 2016 Standard Iso 13485 is designed to be used by organizations involved in the design, production, installation and servicing of medical devices and related services. it can also be used by internal and external parties, such as certification bodies, to help them with their auditing processes. An iso 13485:2016 audit is a comprehensive evaluation process conducted to verify that a medical device manufacturer’s quality management system (qms) meets the requirements of the iso 13485:2016 standard. this standard specifies the requirements for a qms that demonstrate the ability to consistently meet customer and regulatory requirements. Iso 13485 is a well established international standard for quality management systems in the medical device industry. achieving certification demonstrates to potential clients that a company adheres to best practices and regulatory requirements. additionally, implementing iso 13485:2016 can lead to better control over processes, continual. Abstract. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. such organizations can be involved in one or more stages of the life cycle, including.

Sis Certifications On Twitter Iso 13485 2016 Certification Is Iso 13485 is a well established international standard for quality management systems in the medical device industry. achieving certification demonstrates to potential clients that a company adheres to best practices and regulatory requirements. additionally, implementing iso 13485:2016 can lead to better control over processes, continual. Abstract. iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. such organizations can be involved in one or more stages of the life cycle, including.

Iso 13485 2016 Certification вђ Apis Assay Technologies Ltd

Comments are closed.