Selectivity Of Halogen Substitution

13 Halogen Substitution Increases Site Selectivity A 3 H Ach The factors governing selectivity in halogenation of alkanes follow: 1. the rates at which the various c − h c − h bonds of 2 methylbutane are broken by attack of chlorine atoms approach 1:1:1 as the temperature is raised above 300o 300 o. at higher temperatures both chlorine atoms and hydrocarbons become more reactive because of increases. Further experiments showed that 3º hydrogens are about 5 times more toward halogen atoms 1º hydrogens. thus, light induced chlorination of 2 methylpropane gave predominantly (65%) 2 chloro 2 methylpropane, the substitution product of the sole 3º hydrogen, despite the presence of nine 1º hydrogens in the molecule.

Selectivity Of Halogen Substitution Youtube Halogenation is the replacement of one or more hydrogen atoms in an organic compound by a halogen (fluorine, chlorine, bromine or iodine). unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in which a c h bond is broken and a new c x bond is formed. First, by selectivity, we are referring to the regioselectivity of the radical halogenation. for example, can we mix propane with a halogen and selectively substitute the hydrogen on carbon 1 or 2 while leaving the other positions intact: the answer is yes, it is possible, but it only works effectively when bromine is used. for example, when. As we’d also mentioned earlier, this is also the ratio of c–h bond strengths: ch 4 > r–ch 3 > r–ch 2 –r > r 3 c–h . the more stable the free radical that is left behind, the weaker will be the c–h bond strength. 2. secondary c–h bonds are weaker than primary c–h bonds. breaking a secondary c–h bond results in a more stable. Selectivity of halogen bonds on cu(111) and on au(111) the effects of aromatic fluorine substitution on the strengths of halogen bonding interactions involving chlorine, bromine, and iodine. j.
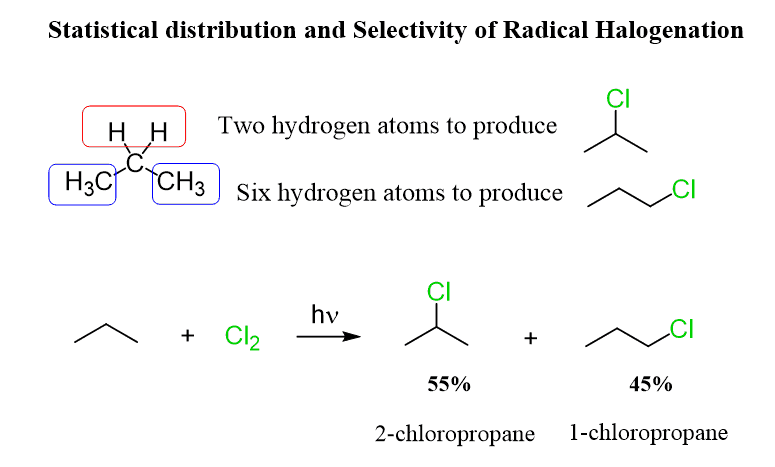
Selectivity In Radical Halogenation With Practice Problems Chemistry As we’d also mentioned earlier, this is also the ratio of c–h bond strengths: ch 4 > r–ch 3 > r–ch 2 –r > r 3 c–h . the more stable the free radical that is left behind, the weaker will be the c–h bond strength. 2. secondary c–h bonds are weaker than primary c–h bonds. breaking a secondary c–h bond results in a more stable. Selectivity of halogen bonds on cu(111) and on au(111) the effects of aromatic fluorine substitution on the strengths of halogen bonding interactions involving chlorine, bromine, and iodine. j. The discussion covers hydrogen atom transfer, halogen atom transfer and homolytic aromatic substitution. nature synthesis polar effects permeate radical chemistry and control the outcome of. The first step is the initiation step. we make new radicals in this step where we had no radicals before. typically, we’ll have the halogen that we want to use in this reaction split into two radicals upon heating it or using the high intensity light. this forms a couple of free radicals.

Investigation Of Enhanced Am Selectivity For Eu In Solvent Extraction The discussion covers hydrogen atom transfer, halogen atom transfer and homolytic aromatic substitution. nature synthesis polar effects permeate radical chemistry and control the outcome of. The first step is the initiation step. we make new radicals in this step where we had no radicals before. typically, we’ll have the halogen that we want to use in this reaction split into two radicals upon heating it or using the high intensity light. this forms a couple of free radicals.

Halogenation Definition Halogenation Types Reactions Importance

Comments are closed.