Second Law Of Thermodynamics Heat Energy Entropy Spontaneous Processes

Second Law Of Thermodynamics Heat Energy Entropy Spontaneousо The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process; it never decreases. an important implication of this law is that heat transfers energy spontaneously from higher to lower temperature objects, but never spontaneously in the reverse direction. This physics video tutorial provides a basic introduction into the second law of thermodynamics. it explains why heat flows from a hot object to a cold obje.
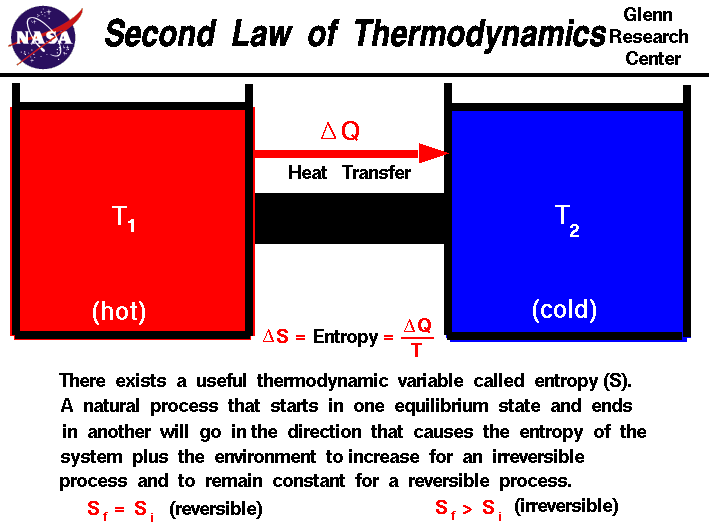
Second Law Of Thermodynamics Recognizing that the work done in a reversible process at constant pressure is w rev = −pΔv, we can express equation 19.2.1 as follows: Δu = qrev wrev = tΔs − pΔv (19.2.3) (19.2.4) thus the change in the internal energy of the system is related to the change in entropy, the absolute temperature, and the pv work done. These results lead to a profound statement regarding the relation between entropy and spontaneity known as the second law of thermodynamics: all spontaneous changes cause an increase in the entropy of the universe. a summary of these three relations is provided in table 19.4.1. The second law of thermodynamics: a law stating that states that the entropy of an isolated system never decreases, because isolated systems spontaneously evolve toward thermodynamic equilibrium—the state of maximum entropy. equivalently, perpetual motion machines of the second kind are impossible. heat engine: any device which converts heat. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. it predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. for example, the.
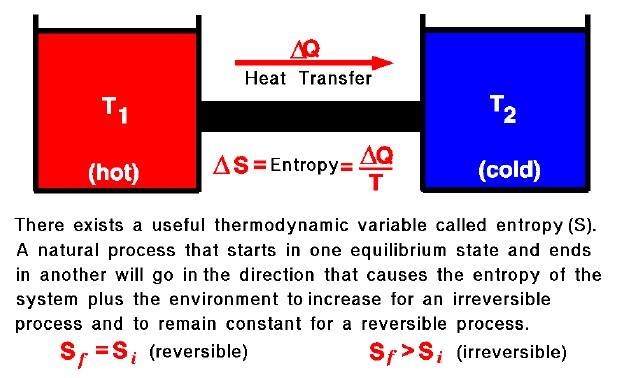
Second Law Entropy Glenn Research Center Nasa The second law of thermodynamics: a law stating that states that the entropy of an isolated system never decreases, because isolated systems spontaneously evolve toward thermodynamic equilibrium—the state of maximum entropy. equivalently, perpetual motion machines of the second kind are impossible. heat engine: any device which converts heat. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. it predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. for example, the. There is yet another way of expressing the second law of thermodynamics. this version relates to a concept called entropy.by examining it, we shall see that the directions associated with the second law—heat transfer from hot to cold, for example—are related to the tendency in nature for systems to become disordered and for less energy to be available for use as work. Example 10.5.1. lets start with an easy reaction: 2h2 (g) o2 (g) → 2h2o (g) the enthalpy, Δh, for this reaction is 241.82 kj, and the entropy, Δs, of this reaction is 233.7 j k. if the temperature is at 25º c, then there is enough information to calculate the standard free energy change, Δg.

Second Law Of Thermodynamics In Terms Of Entropy There is yet another way of expressing the second law of thermodynamics. this version relates to a concept called entropy.by examining it, we shall see that the directions associated with the second law—heat transfer from hot to cold, for example—are related to the tendency in nature for systems to become disordered and for less energy to be available for use as work. Example 10.5.1. lets start with an easy reaction: 2h2 (g) o2 (g) → 2h2o (g) the enthalpy, Δh, for this reaction is 241.82 kj, and the entropy, Δs, of this reaction is 233.7 j k. if the temperature is at 25º c, then there is enough information to calculate the standard free energy change, Δg.

Comments are closed.