Phase Ii And Iii Clinical Trials Evaluating Approved And Download

Phase Ii And Iii Clinical Trials Evaluating Approved And Download Key points of phase iii clinical trials. most phase iii clinical trials include a large number of patients, at least several hundred. these studies are often done in many places across the country (or even around the world) at the same time. phase iii clinical trials are more likely to be offered in local community hospitals and doctor's offices. An overview of phase ii clinical trial designs. clinical trials are studies to test new treatments in humans. typically, these treatments are evaluated over several phases to assess their safety and efficacy. phase i trials are designed to evaluate the safety and tolerability of a new treatment, typically with a small number of patients (e.g.

Phase Ii And Iii Clinical Trials Evaluating Approved And Download Historically, in evaluations of evidence by stakeholders such as regulators, health technology assessment bodies and clinical guideline developers, more emphasis is placed on randomized controlled phase iii trials rather than on phase ii trials.1, 2 however, approvals based on only nonrandomized, uncontrolled phase ii trials are becoming more. Phase ii trials are also important to collect additional safety data, determining drug dosing ranges, routes and timing for phase iii trials, as well as common short term adverse events. there are numerous phase ii trials evaluating the efficacy of pharmacological agents in plp, for example, gabapentin ( 17 ), ketamine ( 18 ), memantine ( 19. Activating human epidermal growth factor receptor 2 (her2) mutations account for approximately 2 4% of non small cell lung cancer (nsclc), but there are no first line her2 targeted therapies currently approved for patients (pts) with locally advanced or metastatic nsclc harboring her2 activating mutations. bay 2927088 is an oral, reversible tyrosine kinase inhibitor that targets her2 and. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before.

Phase Ii And Iii Clinical Trials Evaluating Approved And Download Activating human epidermal growth factor receptor 2 (her2) mutations account for approximately 2 4% of non small cell lung cancer (nsclc), but there are no first line her2 targeted therapies currently approved for patients (pts) with locally advanced or metastatic nsclc harboring her2 activating mutations. bay 2927088 is an oral, reversible tyrosine kinase inhibitor that targets her2 and. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. The focus of this review is to critically evaluate the safety and efficacy outcomes of the most recent, placebo controlled phase i ii clinical studies that enrolled a larger number of patients, in. Abstract. after the success of phase ii development, it comes to the phase iii development. phase iii has fundamental objectives in confirming clinical efficacy and investigating no unacceptable safety concerns. clinical efficacy generally includes effects that are measurable as reflected by clinical outcome assessments such as patient reported.
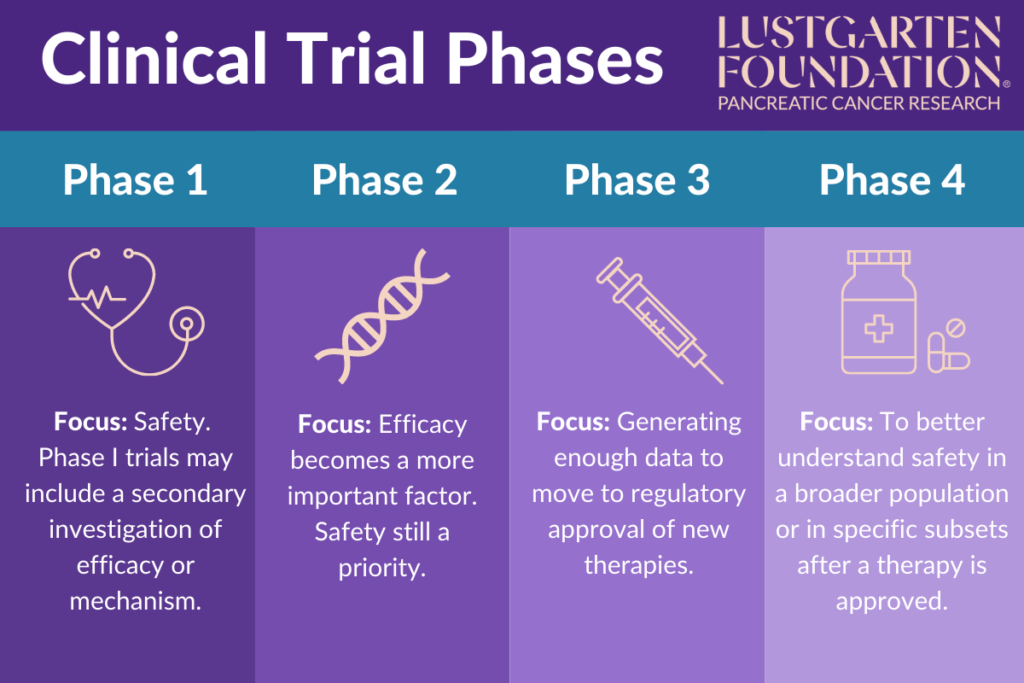
Clinical Trial Phases The focus of this review is to critically evaluate the safety and efficacy outcomes of the most recent, placebo controlled phase i ii clinical studies that enrolled a larger number of patients, in. Abstract. after the success of phase ii development, it comes to the phase iii development. phase iii has fundamental objectives in confirming clinical efficacy and investigating no unacceptable safety concerns. clinical efficacy generally includes effects that are measurable as reflected by clinical outcome assessments such as patient reported.

Phase Ii And Iii Clinical Trials Evaluating Approved And Download

Comments are closed.