Phase I Clinical Trials Overview Dana Farber Cancer Institute

Dana Farber Cancer Institute Harvard Medical School Clinical trials resources at dana farber’s longwood campus. take a virtual tour of dana farber’s longwood medical area campus, and learn about the assets available and our means for conducting clinical trials through our research collaborations, departments, and facilities. view the many spaces and resources where our expert faculty and. Andrew wolanski, np. in phase i clinical trials, investigators evaluate how often and how much of the drug should be given. these early trials are often small, enrolling between 15 and 100 patients, but are an essential step in the development of more effective cancer treatments. “people who enroll in phase i trials are helping us discover.

Phase I Clinical Trials Overview Dana Farber Cancer Institute Youtube For more information about breast cancer clinical trials and treatment at dana farber cancer institute visit dana farber.org adult care treatment. Clinical trials are scientific studies in which new treatments – drugs, diagnostic procedures, and other therapies – are tested in patients to determine if they are safe and effective. search for dana farber clinical trials by cancer type or protocol number. Ref: protocol v9.0, dated 7nov2023. nous 209 01 is a multicenter, open label, multiple cohorts, clinical study, designed to evaluate safety, tolerability, and immunogenicity, and to detect any preliminary evidence of anti tumor activity of nous 209 genetic polyvalent vaccine plus pembrolizumab combination therapy in adult subjects with unresectable or metastatic deficient mismatch repair (dmmr. Geoffrey shapiro, md, phd, is director of dana farber cancer institute’s early drug development center (eddc), which specializes in conducting phase i clinical trials: small, carefully designed safety studies of experimental drugs being given to patients for the first time. here, he answers common questions about clinical trials and how they.

How Do I Find The Right Clinical Trial For Me Dana Farber Cancer Ref: protocol v9.0, dated 7nov2023. nous 209 01 is a multicenter, open label, multiple cohorts, clinical study, designed to evaluate safety, tolerability, and immunogenicity, and to detect any preliminary evidence of anti tumor activity of nous 209 genetic polyvalent vaccine plus pembrolizumab combination therapy in adult subjects with unresectable or metastatic deficient mismatch repair (dmmr. Geoffrey shapiro, md, phd, is director of dana farber cancer institute’s early drug development center (eddc), which specializes in conducting phase i clinical trials: small, carefully designed safety studies of experimental drugs being given to patients for the first time. here, he answers common questions about clinical trials and how they. Clinical trials are carried out in three phases: phase 1 trials are the first to evaluate a drug in humans. a small number of patients — usually around 20 to 100 — are given the drug, first in very low doses and then escalating to find the dose of the treatment that can be given safely without causing severe side effects. phase 2 trials. The dana farber harvard cancer center (df hcc) is one of twelve lead academic organizations (laos) selected to be part of the national cancer institute (nci) experimental therapeutics clinical trials network (etctn). df hcc investigators utilize their experience and expertise in clinical drug development to contribute to the etctn’s.
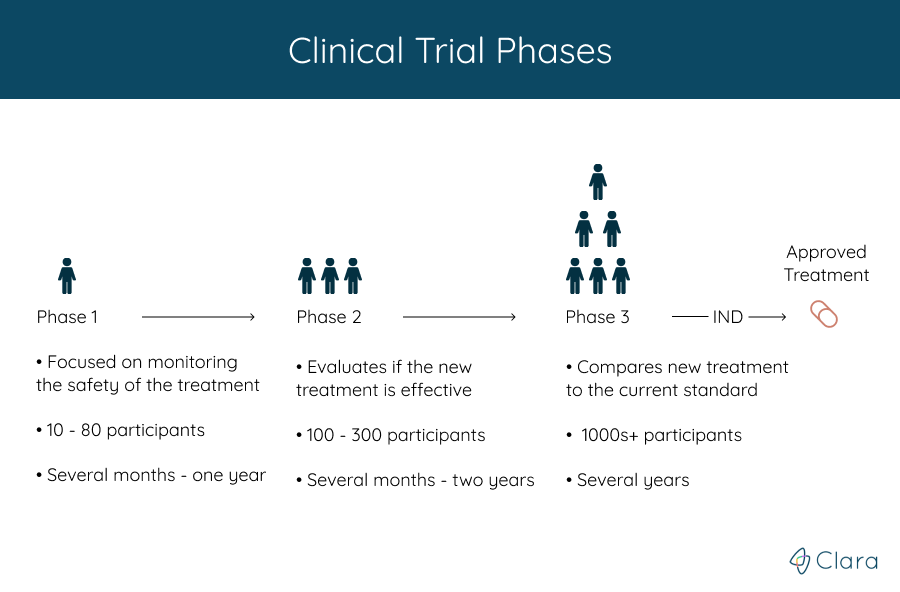
Clinical Trial Phases 1 2 3 4 Find Fda Clinical Trial Phases Clinical trials are carried out in three phases: phase 1 trials are the first to evaluate a drug in humans. a small number of patients — usually around 20 to 100 — are given the drug, first in very low doses and then escalating to find the dose of the treatment that can be given safely without causing severe side effects. phase 2 trials. The dana farber harvard cancer center (df hcc) is one of twelve lead academic organizations (laos) selected to be part of the national cancer institute (nci) experimental therapeutics clinical trials network (etctn). df hcc investigators utilize their experience and expertise in clinical drug development to contribute to the etctn’s.

Comments are closed.