Multiple Phases Of Clinical Trial With Outcomes Clinical Research Trialођ
Multiple Phases Of Clinical Trial With Outcomes Clinical Tria Abstract. selecting outcomes for clinical trials requires a wide range of considerations relating to clinical interpretation, ethics relating to therapy effectiveness and safety, and statistical optimality of measures. appropriate outcome choice plays a key role in determining the usefulness and or success of a study and can affect whether a. If these assumptions are not met, the resulting estimates of treatment efficacy are difficult to interpret. 6 various recent clinical studies have used multiple event time outcomes as their end points . the methods used in these trials all require some degree of modeling, and their modeling assumptions can be difficult to justify empirically.

Multiple Phases Of Clinical Trial With Outcomes Clinical Rese This article tackles both practical and statistical issues in the handling of multiple outcomes in clinical trials, with relevance to trial design, analysis, and reporting. specific topics illustrated by examples include: the advantage of prespecifying priorities amongst outcomes and analyses, corre …. Additional guidance for using multiple outcomes and analyses from multiple reports of clinical trials is needed. acknowledgments the authors used the open access database, systematic review data repository (srdr), and are grateful to jens jap, bryant smith, and joseph lau for making this possible without charge and for the support they provided. Multilevel models have become a standard data analysis approach in intervention research. although the vast majority of intervention studies involve multiple outcome measures, few studies use multivariate analysis methods. the authors discuss multivariate extensions to the multilevel model that can be used by psychotherapy researchers. Clinical trials: design, endpoints and interpretation of outcomes. megan othus, mei jie zhang &. robert peter gale. bone marrow transplantation 57, 338–342 (2022) cite this article. 15k accesses.

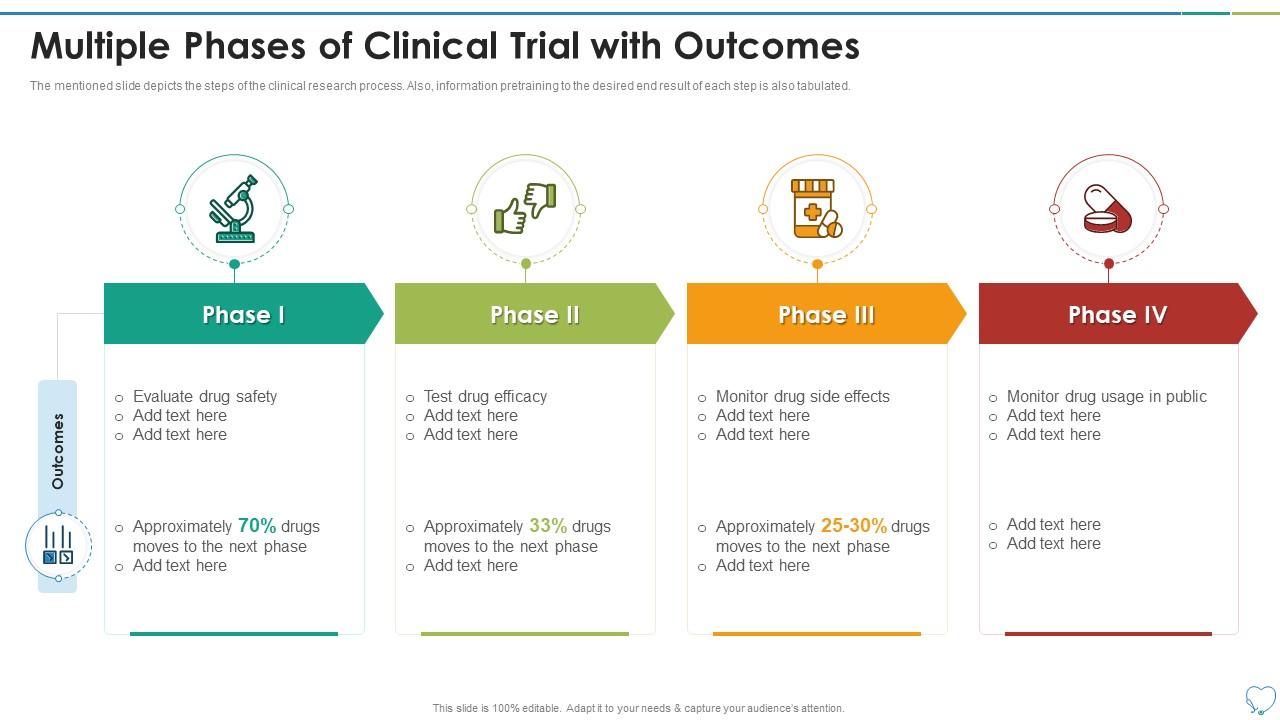
Multiple Phases Of Clinical Trial With Outcomes Research Design Multilevel models have become a standard data analysis approach in intervention research. although the vast majority of intervention studies involve multiple outcome measures, few studies use multivariate analysis methods. the authors discuss multivariate extensions to the multilevel model that can be used by psychotherapy researchers. Clinical trials: design, endpoints and interpretation of outcomes. megan othus, mei jie zhang &. robert peter gale. bone marrow transplantation 57, 338–342 (2022) cite this article. 15k accesses. Abstract. the purpose of late phase clinical trials is to generate evidence of sufficient validity and generalisability to be translated into practice and policy to improve health outcomes. it is therefore crucial that the chosen endpoints are meaningful to the clinicians, patients and policymakers that are the end users of evidence generated. Are conducted in multiple phases to ensure safety and efficacy, and today, we’ll discuss phase 4 clinical trials in depth. the has resulted in highly structured and regulated systems. in fact, before trials can reach phase 4, they must first go through the following phases: phase 1: when a clinical trial begins, a new drug is tested on a few.

Comments are closed.