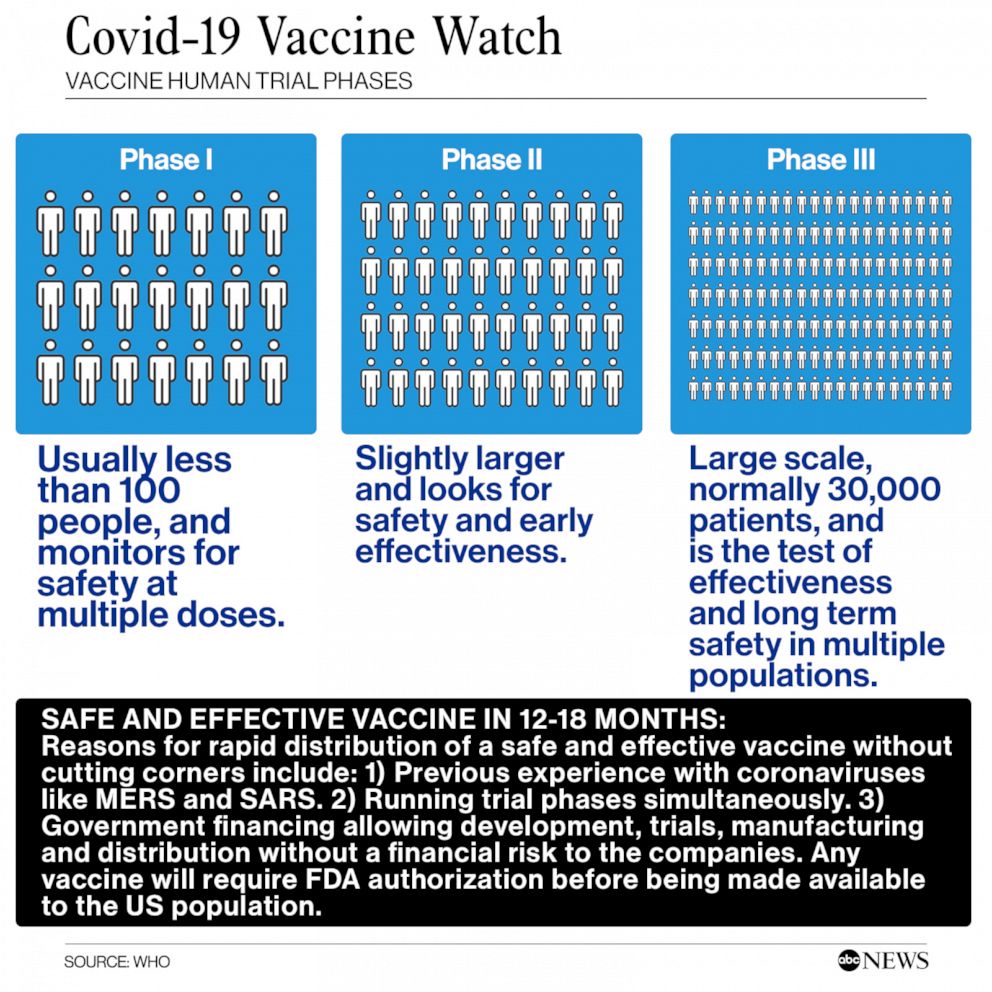
Moderna Coronavirus Vaccine Trial Enters Phase 3 Of Testing
Human Trial For Coronavirus Vaccine Launched By Moderna Enters Phas Cambridge, ma accesswire march 26, 2024 moderna, inc. (nasdaq:mrna) today announced that mrna 1283, the company's next generation covid 19 vaccine, has successfully met the primary endpoints of its phase 3 clinical trial, demonstrating a higher immune response against sars cov 2 when compared to mrna 1273.222, moderna's licensed covid 19. The phase 3 trial of mrna 1273, a lipid nanoparticle–encapsulated mrna expressing the prefusion stabilized spike glycoprotein of sars cov 2, 11 showed a 94.1% vaccine efficacy against covid 19.
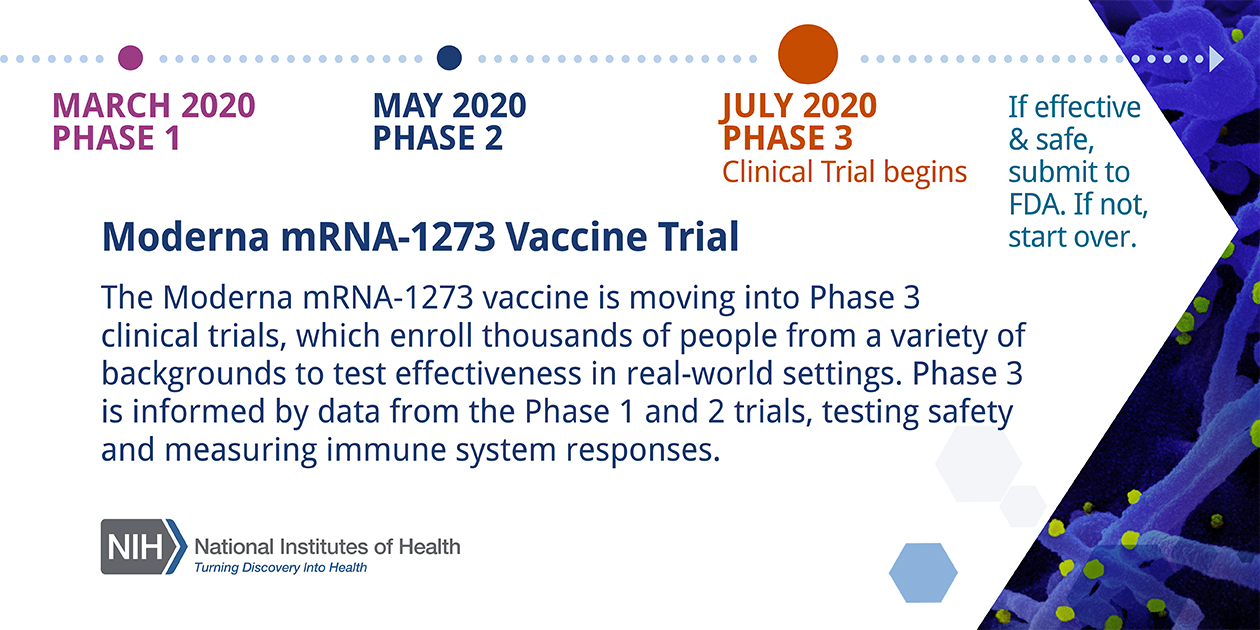
Multimedia National Institutes Of Health Nih The coronavirus efficacy (cove) phase 3 trial was launched in late july 2020 to assess the safety and efficacy of the mrna 1273 vaccine in preventing sars cov 2 infection. an independent data and. The investigational vaccine directs the body’s cells to express the spike protein to elicit a broad immune response. a phase 1 clinical trial found the candidate vaccine to be safe, generally well tolerated and able to induce antibodies with high levels of virus neutralizing activity. moderna initiated phase 2 testing of the vaccine in may 2020. The company has received close to $1 billion in federal funding from the biomedical advanced research and development authority to develop its vaccine and complete its final phase of testing. Here's how it works. the biotech firm's leading candidate has just entered phase three clinical trials in the u.s. find out why this vaccine technology is promising—but not without its skeptics.

Hear From First Us Participant To Receive Moderna Phase 3 Trial Vaccineођ The company has received close to $1 billion in federal funding from the biomedical advanced research and development authority to develop its vaccine and complete its final phase of testing. Here's how it works. the biotech firm's leading candidate has just entered phase three clinical trials in the u.s. find out why this vaccine technology is promising—but not without its skeptics. What. the investigational vaccine known as mrna 1273 was 94.1% efficacious in preventing symptomatic coronavirus disease 2019 (covid 19), according to preliminary results from a phase 3 clinical trial reported in the new england journal of medicine. the vaccine also demonstrated efficacy in preventing severe covid 19. As part of the arrangement under ows, representatives from niaid, barda and moderna are part of the oversight group that receives recommendations from the trial’s independent dsmb. the same dsmb also oversees the additional ows supported phase 3 clinical trials evaluating covid 19 vaccine candidates.

Comments are closed.