Ionic Bonding What Causes An Atom To Form Ionic Bonds

Ionic Bond Facts Definition Properties Examples Diagrams Modified by joshua halpern (howard university) 8.2: ionic bonding is shared under a cc by nc sa 3.0 license and was authored, remixed, and or curated by libretexts. the amount of energy needed to separate a gaseous ion pair is its bond energy. the formation of ionic compounds are usually extremely exothermic. The atom that loses electrons becomes a positively charged ion, known as a cation. the atom that gains electrons becomes a negatively charged ion, known as an anion. when the two ions combine via ionic bond, they form ionic compounds. the types of elements forming ionic bonds are metals and nonmetals [1 4].
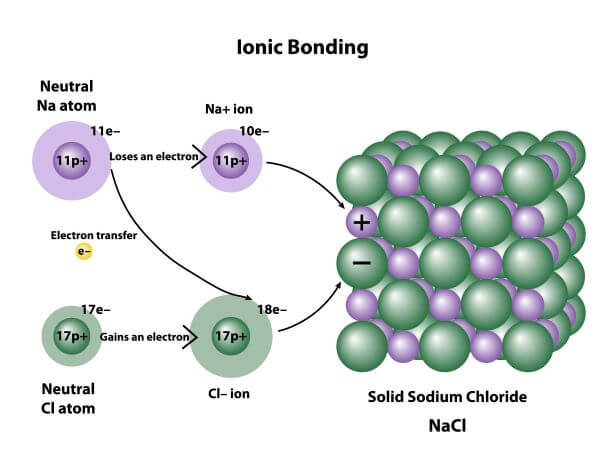
Ionic Bond Examples Biology Dictionary Chlorine is poisonous, but sodium chloride is essential to life; sodium atoms react vigorously with water, but sodium chloride simply dissolves in water. figure 4.1.1 4.1. 1: (a) sodium is a soft metal that must be stored in mineral oil to prevent reaction with air or water. (b) chlorine is a pale yellow green gas. This often causes ionic compounds to be very stable. ionic bonds have high bond energy. bond energy is the mean amount of energy required to break the bond in the gaseous state. most ionic compounds exist in the form of a crystal structure, in which the ions occupy the corners of the crystal. such a structure is called a crystal lattice. An ionic bond is actually the extreme case of a polar covalent bond, the latter resulting from unequal sharing of electrons rather than complete electron transfer. ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. An ionic solid is formed out of endlessly repeating patterns of ionic pairs. because we want to establish the basics about ionic bonding and not get involved in detail we will continue to use table salt, nacl, to discuss ionic bonding. in nacl, of course, an electron is transferred from each sodium atom to a chlorine atom leaving na and cl.
.PNG)
Ionic Bonding Presentation Chemistry An ionic bond is actually the extreme case of a polar covalent bond, the latter resulting from unequal sharing of electrons rather than complete electron transfer. ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. An ionic solid is formed out of endlessly repeating patterns of ionic pairs. because we want to establish the basics about ionic bonding and not get involved in detail we will continue to use table salt, nacl, to discuss ionic bonding. in nacl, of course, an electron is transferred from each sodium atom to a chlorine atom leaving na and cl. The properties of ionic compounds shed some light on the nature of ionic bonds. ionic solids exhibit a crystalline structure and tend to be rigid and brittle; they also tend to have high melting and boiling points, which suggests that ionic bonds are very strong. ionic solids are also poor conductors of electricity for the same reason—the. 5.1 ionic bonding. as you have learned in chapter 2, ions are atoms or molecules bearing an electrical charge. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell.

Ionic Bond Definition Properties Examples Facts Britannica The properties of ionic compounds shed some light on the nature of ionic bonds. ionic solids exhibit a crystalline structure and tend to be rigid and brittle; they also tend to have high melting and boiling points, which suggests that ionic bonds are very strong. ionic solids are also poor conductors of electricity for the same reason—the. 5.1 ionic bonding. as you have learned in chapter 2, ions are atoms or molecules bearing an electrical charge. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell.

Comments are closed.