Identifying Phase Transitions On A Heating Curve Chemistry Study

Identifying Phase Transitions On A Heating Curve Chemistry Study Step 1: correlate states of matter to different phase transitions. melting involves the transition of a solid to a liquid. step 2: label the different points on the heating curve that corresponds. Figure \(\pageindex{1}\): a typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. plateaus in the curve (regions of constant temperature) are exhibited when the substance undergoes phase transitions. consider the example of heating a pot of water to boiling.
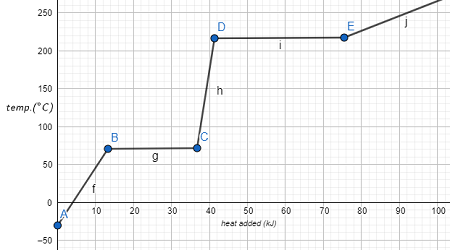
Identifying Phase Transitions On A Heating Curve Chemistry Study Practice identifying phase transitions on a heating curve with practice problems and explanations. get instant feedback, extra help and step by step explanations. boost your chemistry grade with. This video is my attempt at providing a simple but in depth explanation of this aleks chemistry topic as i walk you through the steps necessary to solve the. Figure \(\pageindex{8}\) shows a typical heating curve. figure \(\pageindex{8}\): a typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. plateaus in the curve (regions of constant temperature) are exhibited when the substance undergoes phase transitions. A heating curve depicts the amount of energy added or subtracted on the x axis and the temperature of the substance on the y axis. when determining the q for a heating curve, the phase transition line segments must be calculated separately from the temperature change segments since there are different processes for calculating the q values.

Identifying Phase Transitions On A Heating Curve Chemistry Study Figure \(\pageindex{8}\) shows a typical heating curve. figure \(\pageindex{8}\): a typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. plateaus in the curve (regions of constant temperature) are exhibited when the substance undergoes phase transitions. A heating curve depicts the amount of energy added or subtracted on the x axis and the temperature of the substance on the y axis. when determining the q for a heating curve, the phase transition line segments must be calculated separately from the temperature change segments since there are different processes for calculating the q values. Phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase diagram depicts the phase. Since the normal boiling point is the temperature at which the vapor pressure equals atmospheric pressure at sea level, we know one vapor pressure temperature value (t1 = 80.1 °c = 353.3 k, p1 = 101.3 kpa, Δ hvap = 30.8 kj mol) and want to find the temperature (t2) that corresponds to vapor pressure p2 = 83.4 kpa.
Identifying Phase Transitions On A Heating Curve Practice Chemistry Phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase diagram depicts the phase. Since the normal boiling point is the temperature at which the vapor pressure equals atmospheric pressure at sea level, we know one vapor pressure temperature value (t1 = 80.1 °c = 353.3 k, p1 = 101.3 kpa, Δ hvap = 30.8 kj mol) and want to find the temperature (t2) that corresponds to vapor pressure p2 = 83.4 kpa.

Comments are closed.