How To Write The Orbital Diagram For Seaborgium Sg
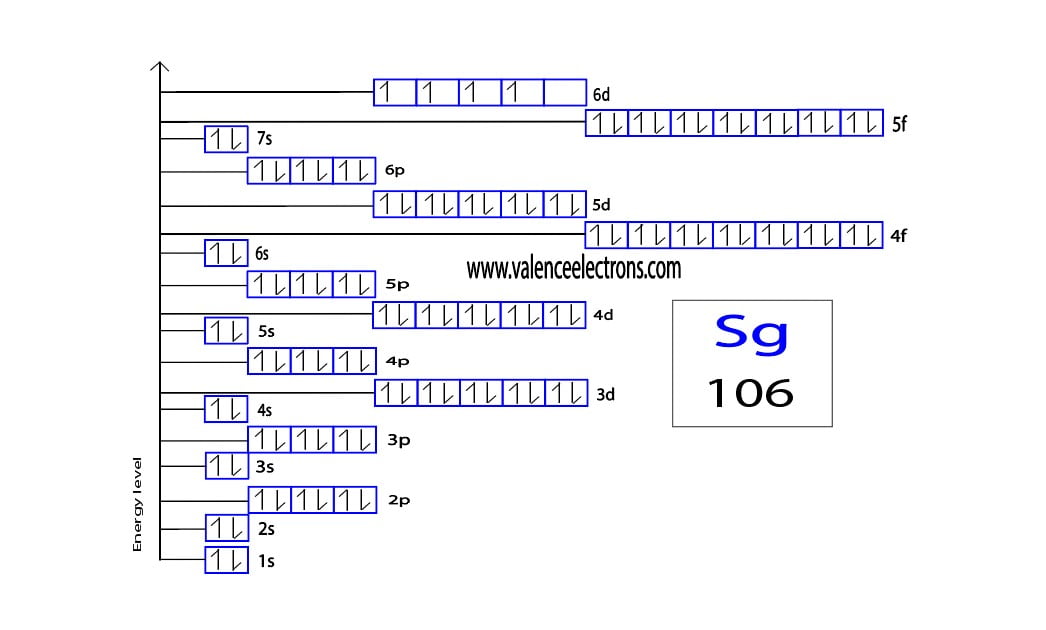
How To Write The Orbital Diagram For Seaborgium Sg The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the bohr atomic model. the electron configuration of all the elements can be done through the orbital diagram. electron configuration of seaborgium through orbital. atomic energy shells are subdivided into sub energy levels. The lowest energy orbitals are closest to the nucleus and the higher energy orbitals are progressively further away from the nucleus in order of their energy levels. to write the orbital diagram of seaborgium, you have to write the orbital notation of seaborgium. which has been discussed in detail above.
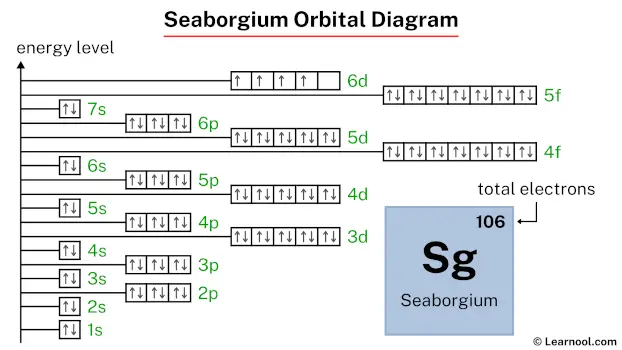
Seaborgium Electron Configuration Learnool Start writing electron configuration from the very first element (i.e., hydrogen) all the way up to seaborgium. start from 1s and write till sg for full electron configuration | image: learnool so the electron configuration of seaborgium will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 4 . The ratio of the average mass per atom of an isotope to 1 12 the mass of a carbon 12 atom. relative atomic mass is also known as atomic weight (symbol: a r ). seaborgium (sg) has an atomic mass of 106. find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Seaborgium (sg), with 106 electrons, has a complex configuration written as 5f¹⁴ 6d⁴ 7s². here, represents the filled electron shells of radon. the valence. Seaborgium is named for glenn t. seaborg, who was instrumental in producing several transuranium elements. allotropes. sg. seaborgium. 106. [269] glossary. groupa vertical column in the periodic table. members of a group typically have similar properties and electron configurations in their outer shell.

Comments are closed.