Heating Curve Calculation Benzene Youtube
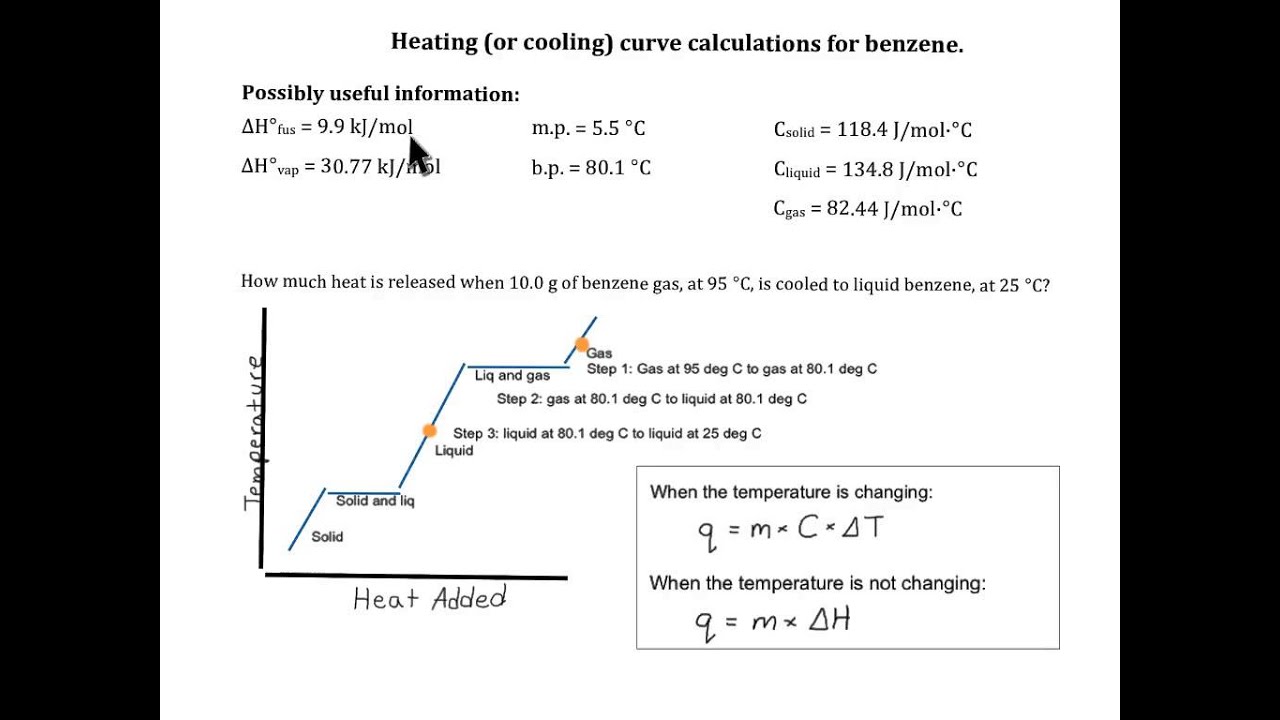
Heating Curve Calculation Benzene Youtube In this example, we use the heating curve of benzene to determine the energy needed to for a temperature and phase change of benzene. how much heat is requi. About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright.

Chemistry 201 Heating Curve Of Benzene Youtube Kentchemistry links matter heatingcurve.htmi take you though the basics of interpreting a heating curve. identifying solid, liquid and gas pha. Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. A heating curve can be used to calculate the enthalpy when a substance is heated. if we were to heat 25.00 g of water from 15.0 °c to 115.0 °c, we can determine Δh for the heating process. below is a heating curve for water from 15.0 °c to 115.0 °c. temperature is on the.

Thermochemistry 12 Heating Curve Calculation 1 Youtube The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. A heating curve can be used to calculate the enthalpy when a substance is heated. if we were to heat 25.00 g of water from 15.0 °c to 115.0 °c, we can determine Δh for the heating process. below is a heating curve for water from 15.0 °c to 115.0 °c. temperature is on the. Heating curves. figure 11.7.3 11.7. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (cs) of. Benzene reactions. 10m. 23. chemistry of the nonmetals 2h 39m. heating curves tutorial: how to calculate enthalpy changes in heating & cooling | crash chemistry.

Heating Curve Calculation Benzene Worksheets Printable Preschool Heating curves. figure 11.7.3 11.7. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (cs) of. Benzene reactions. 10m. 23. chemistry of the nonmetals 2h 39m. heating curves tutorial: how to calculate enthalpy changes in heating & cooling | crash chemistry.

Heating Curve Calculation 11 85 Youtube

Comments are closed.