Heating And Cooling Curve Worksheet 1 Pdf Melting Point Phase
Heating And Cooling Curve Worksheet By Kimberly Frazier Tpt Heating and cooling curve worksheet 1 free download as pdf file (.pdf), text file (.txt) or read online for free. the document is a chemistry worksheet that contains multiple questions about heating and cooling curves. it includes diagrams of heating curves and asks students to interpret the curves to determine phase changes, temperatures of. Heating&cooling curves a)50°c and 3 min b)50°c and 5 min c)110°c and 4 min d)110°c and 14 min 7.starting as a solid, a sample of a substance is heated at a constant rate. the graph below shows the changes in temperature of this sample. what is the melting point of the sample and the total time required to completely melt the sample after it.
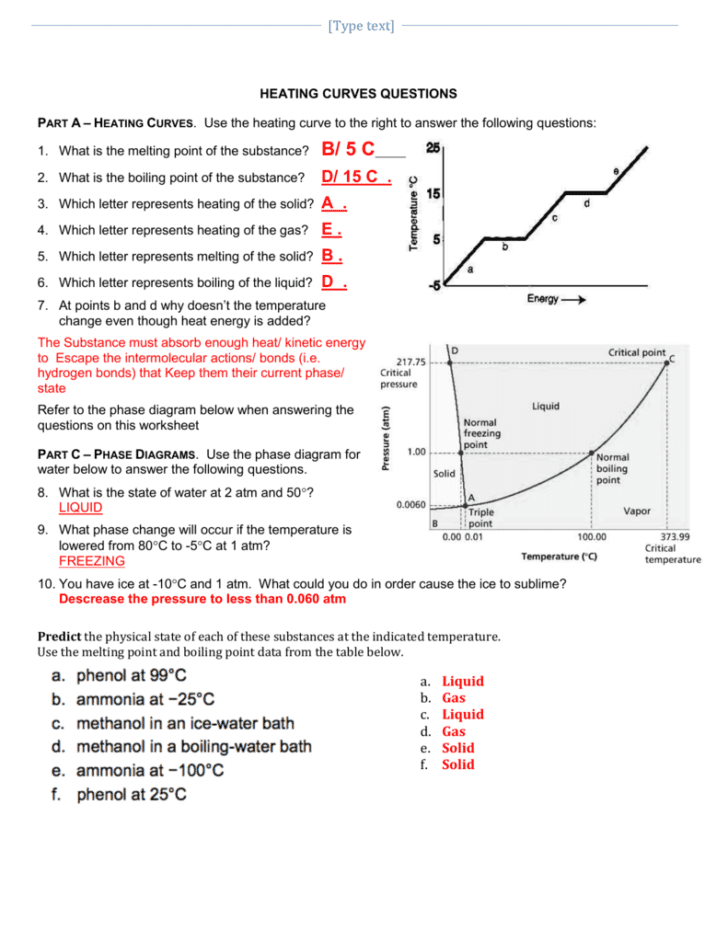
Heating Cooling Curve Worksheet Answers вђ Db Excel Melting point boiling point heat of fusion heat of vapor. cp (solid) cp liquid) cp (vapor) 0.0°c 100.0°c 334 j g 2260 j g 2.05 j gºc 4.18 j gºc 1.90 j gºc the data below are for water (h 2 o) 5. heat needed to raise temperature of water from 100 to 125ºc. determine the heat needed to 15 g of ice at 20ºc to 125ºc. Heating curve worksheet 1 the heating curve shown above is a plot of temperature vs time. it represents the heating of substance x at a constant rate of heat transfer. answer the following questions using this heating curve: 1. in what part of the curve would substance x have a definite shape and definite volume? 2. Name worksheet provided by mr. mellon from pittsfordschools heating and cooling curves & thermal equilibrium practice the curve below was made from data collected at standard pressure. use the graph to answer the following ()s: 1. 2. 3. The general equation for calculating heat energy to change a solid to a liquid is: heat = mass x heat of fusion. q = m lf. calculate the heat necessary to change 10 g of ice(s) at 0°c to 10 g of water(l) at 0°c.(b c) 340 j g) = 3400 jb. liquid phaseliquids have a definite volume, but as.
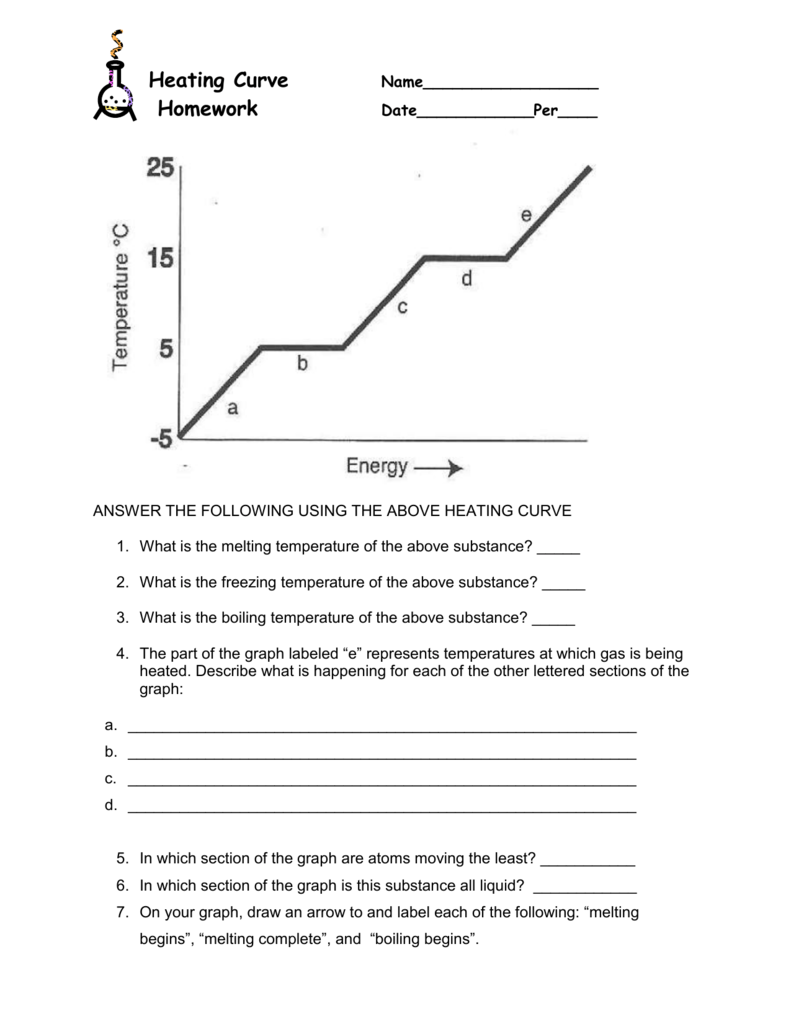
Heating Curve Worksheet Name worksheet provided by mr. mellon from pittsfordschools heating and cooling curves & thermal equilibrium practice the curve below was made from data collected at standard pressure. use the graph to answer the following ()s: 1. 2. 3. The general equation for calculating heat energy to change a solid to a liquid is: heat = mass x heat of fusion. q = m lf. calculate the heat necessary to change 10 g of ice(s) at 0°c to 10 g of water(l) at 0°c.(b c) 340 j g) = 3400 jb. liquid phaseliquids have a definite volume, but as. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. Per: heating curve worksheetheating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools dow. , condenses or freezes. specific heat equation (q=mc∆t) allows us to calculate the energy changes as a substance warms or cools, because there’s a change i.

Comments are closed.