Finding And Calculating An Empirical Formula Of A Compound How To Pass Chemistry
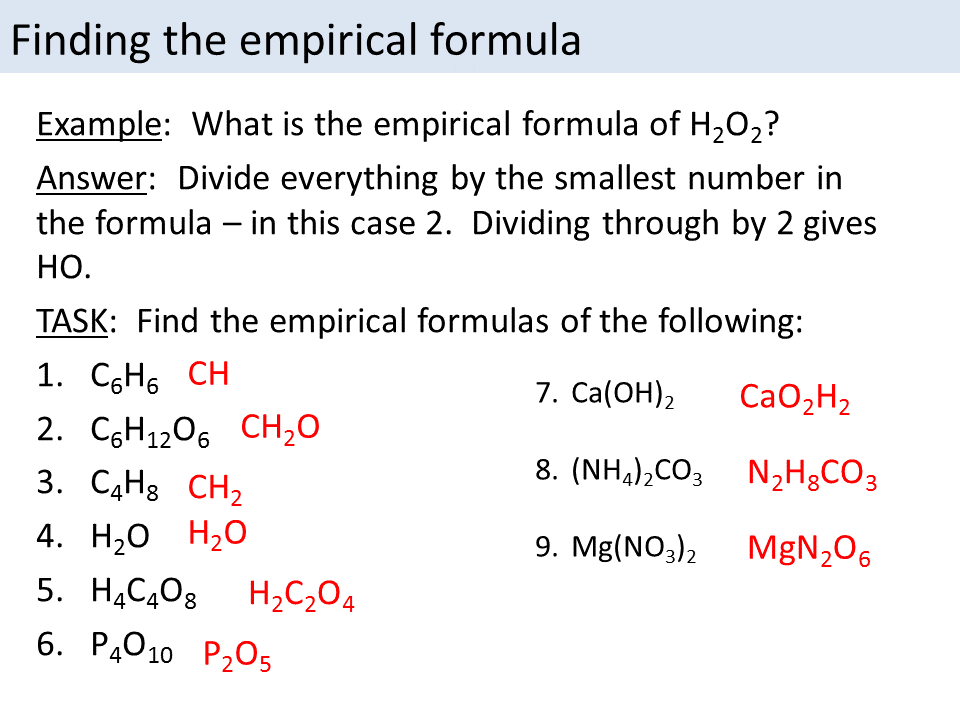
Empirical Formula And Molecular Formula Calculations Gcse 9 1 This video goes into detailed steps on how to find the empirical formula of a compound. hooray for no more confusion!check out my new complete guide on empir. Determining empirical formulas. an empirical formula tells us the relative ratios of different atoms in a compound. the ratios hold true on the molar level as well. thus, h 2 o is composed of two atoms of hydrogen and 1 atom of oxygen. likewise, 1.0 mole of h 2 o is composed of 2.0 moles of hydrogen and 1.0 mole of oxygen.
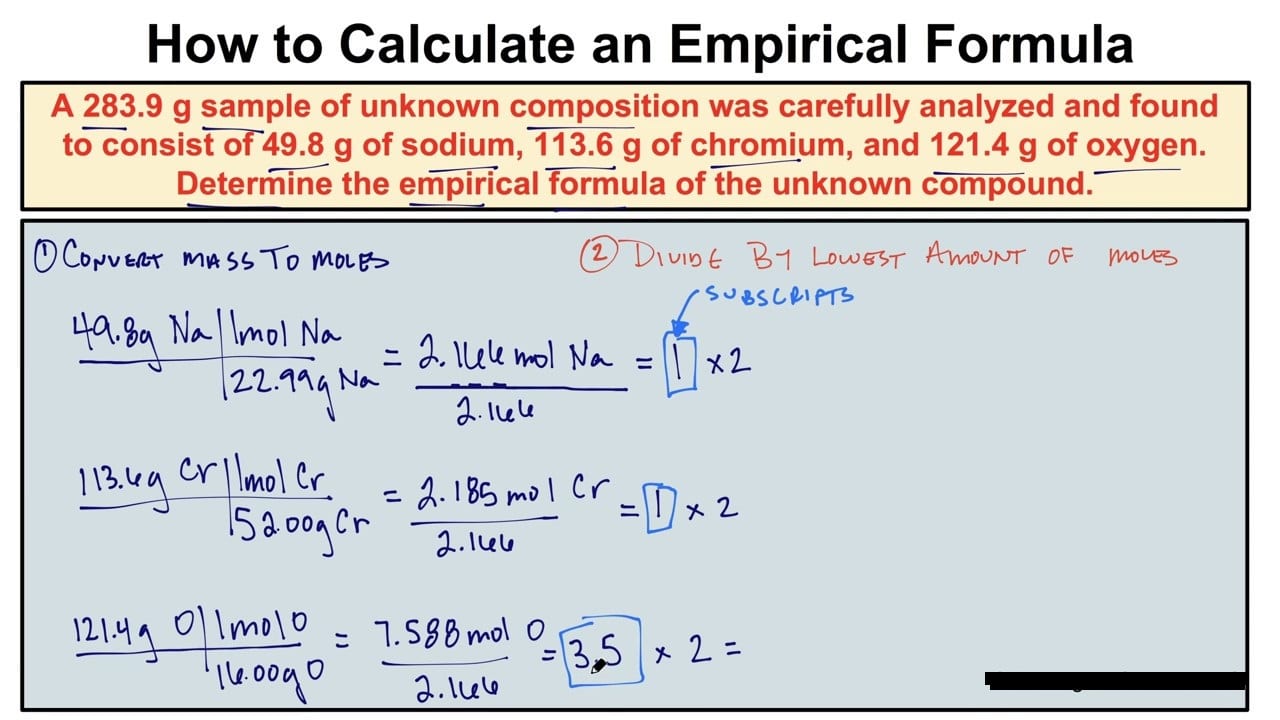
Empirical Formula Definition Calculator Best Examples Get Education Bee Finding and calculating an empirical formula of a compound | how to pass chemistry. The best place to start is to find the smallest number of moles. in this case, it is silver and nitrogen at 0.59 moles. divide each element’s amount by this number. silver: nitrogen: oxygen: for every mole of silver there is one mole of nitrogen and 3 moles of oxygen. the empirical formula is then agno 3. answer:. To find the empirical formula of a compound, we must know the percentage composition of the compound. if you are finding the empirical formula for homework, you will most likely be given the percentages. in a chemistry lab, to find the percentage composition, the compound would be examined through some physical experiments and then quantitative. Derivation of molecular formulas. recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. determining the absolute numbers of atoms that compose a single molecule of a covalent compound requires knowledge of both its empirical formula and its molecular mass or molar mass.

Comments are closed.