Find The Quantity Of Heat Heating Curve Practice Problem

Heating And Cooling Curves Worksheets Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. Multi step problems with changes of state. heating curves show the phase changes that a substance undergoes as heat is continuously absorbed. figure \(\pageindex{1}\): heating curve of water. (cc by nc; ck 12) the specific heat of a substance allows us to calculate the heat absorbed or released as the temperature of the substance changes.
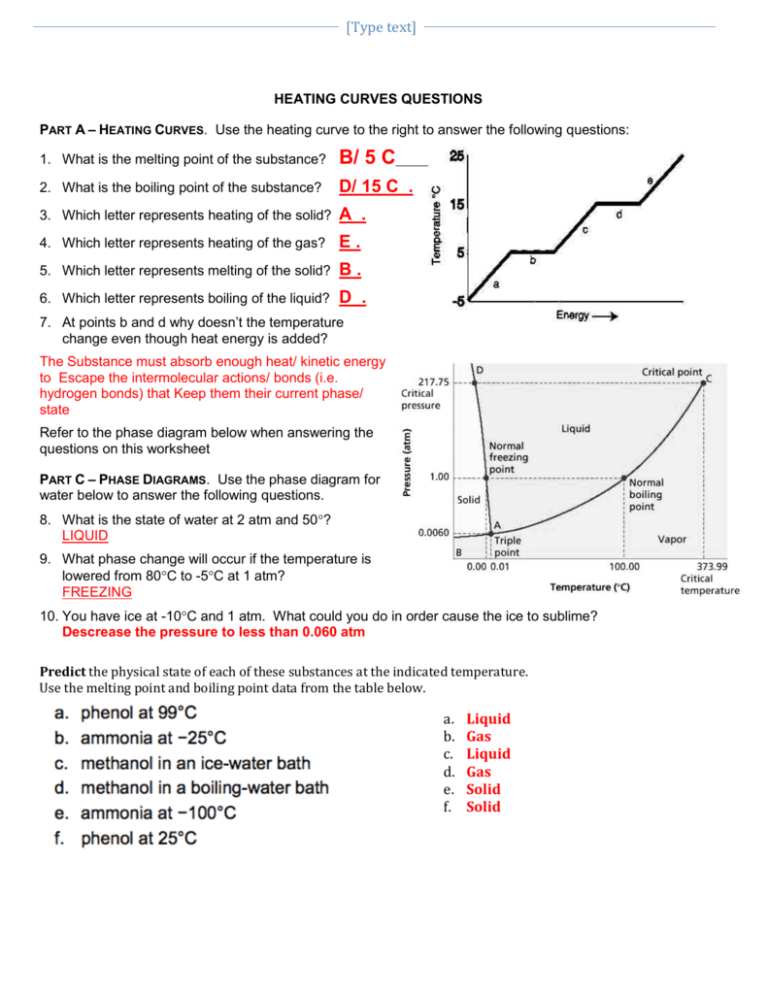
Heating Curves Worksheets The general equation for calculating heat energy to change a solid to a liquid is: heat = mass x heat of fusion. q = m lf. calculate the heat necessary to change 10 g of ice(s) at 0°c to 10 g of water(l) at 0°c.(b c) 340 j g) = 3400 jb. liquid phaseliquids have a definite volume, but as. Problem 8.1.2 8.1. 2. evaporation of sweat requires energy and thus take excess heat away from the body. some of the water that you drink may eventually be converted into sweat and evaporate. if you drink a 20 ounce bottle of water that had been in the refrigerator at 3.8 °c, how much heat is needed to convert all of that water into sweat and. Learn how to solve heating curve problems with this easy to follow video tutorial. you'll see examples, formulas, and tips for chemistry students. Study with quizlet and memorize flashcards containing terms like it's in the solid state. the temperature is going up and the particles are moving faster., it's melting. it's going from solid liquid., it's in the liquid state. the temperature is going up. and more.

Comments are closed.