Fda To Rapidly Work Toward Emergency Use Authorization Of Pfi
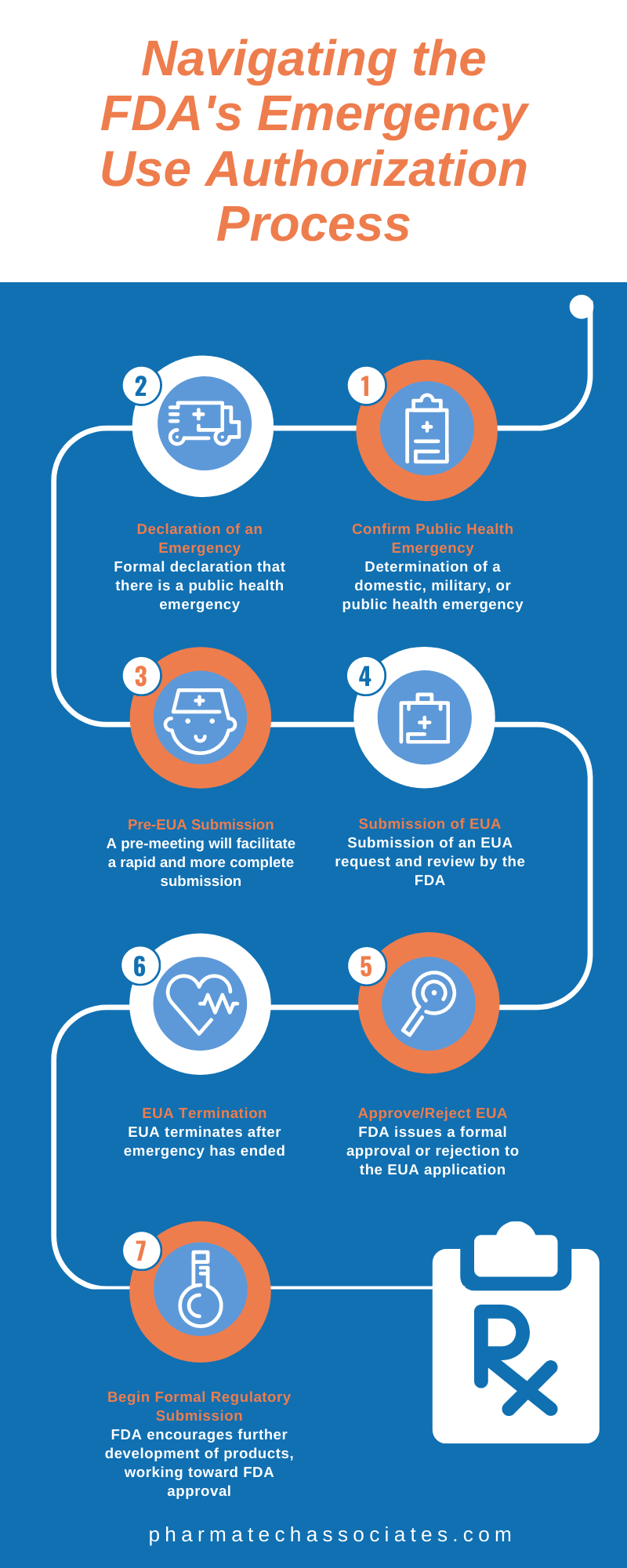
Navigating The юааfdaюабтащs юааemergencyюаб юааuseюаб юааauthorizationюаб Process The u.s. food and drug administration will rapidly work toward finalization and issuance of an emergency use authorization of the pfizer biontech covid 19 vaccine, according to a statement. Fda authorizes pfizer's coronavirus vaccine for emergency use 03:44. the u.s. food and and drug administration said friday it would work quickly to authorize pfizer's covid 19 vaccine for.
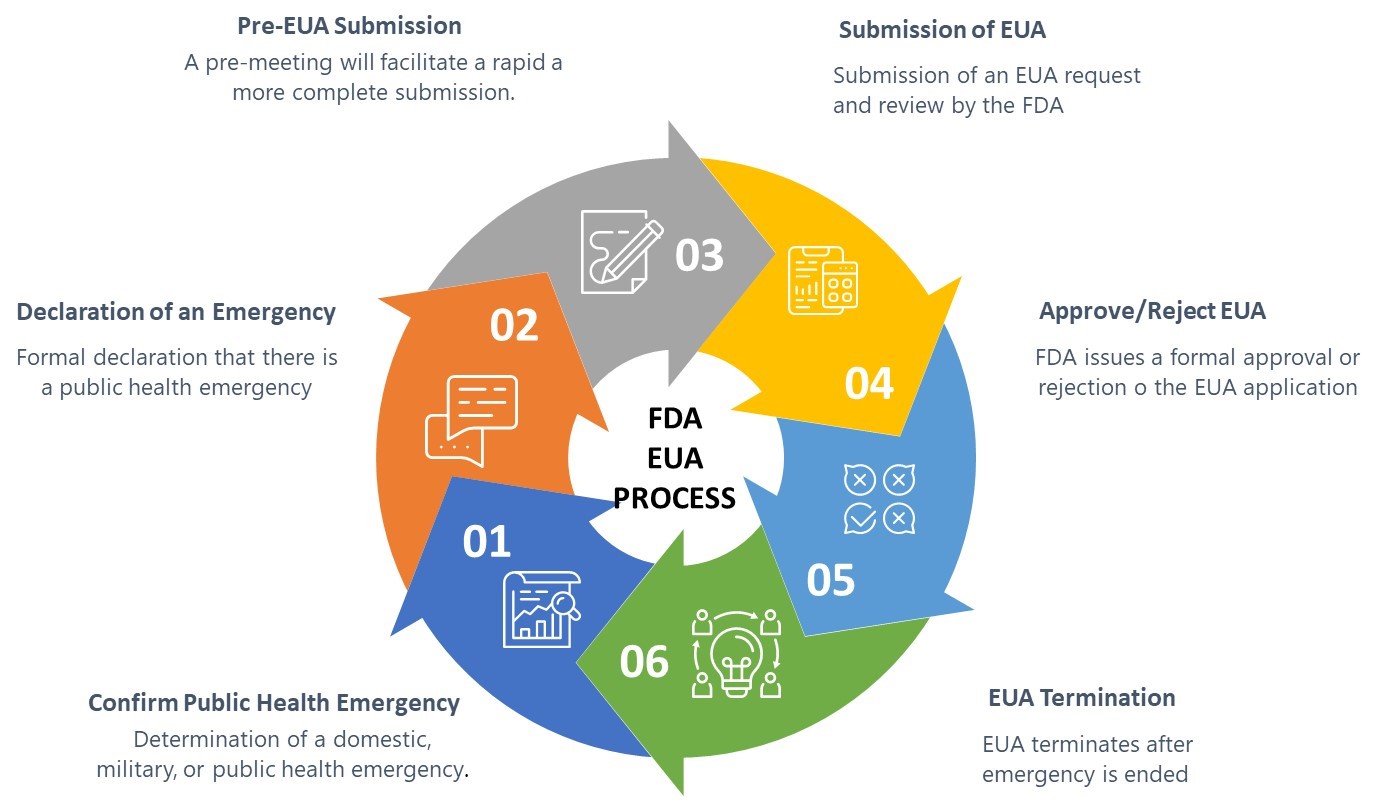
Navigating The юааfdaюабтащs юааemergencyюаб юааuseюаб юааauthorizationюаб Process The u.s. food and and drug administration said friday it will work quickly to authorize pfizer's covid 19 vaccine for emergency use after an advisory panel voted in favor of it. "following. December 11, 2020 at 11:08 a.m. est. this article is free to access. the food and drug administration said early friday that it “will rapidly work toward” authorization of the pfizer biontech. [01 29 2024] in december 2021, fda authorized paxlovid for emergency use for the treatment of adults and pediatric patients (12 years of age and older weighing at least 40 kg) who are at high risk. The food and drug administration said friday it will work rapidly toward finalizing and issuing an emergency use authorization for pfizer covid 19 vaccine following an endorsement of the shot by a.

Fda To Rapidly Work Toward Emergency Use Authorization Of [01 29 2024] in december 2021, fda authorized paxlovid for emergency use for the treatment of adults and pediatric patients (12 years of age and older weighing at least 40 kg) who are at high risk. The food and drug administration said friday it will work rapidly toward finalizing and issuing an emergency use authorization for pfizer covid 19 vaccine following an endorsement of the shot by a. Under section 564 of the federal food, drug, and cosmetic act (fd&c act), when the secretary of hhs declares that an emergency use authorization is appropriate, fda may authorize unapproved. Expanded authorization allows more americans to receive a booster dose to help preserve a high level of protection against covid 19 pfizer inc. (nyse: pfe) and biontech se (nasdaq: bntx) today announced that the u.s. food and drug administration (fda) has expanded the emergency use authorization (eua) of a booster dose of the pfizer biontech covid 19 vaccine to include individuals 18 years of.
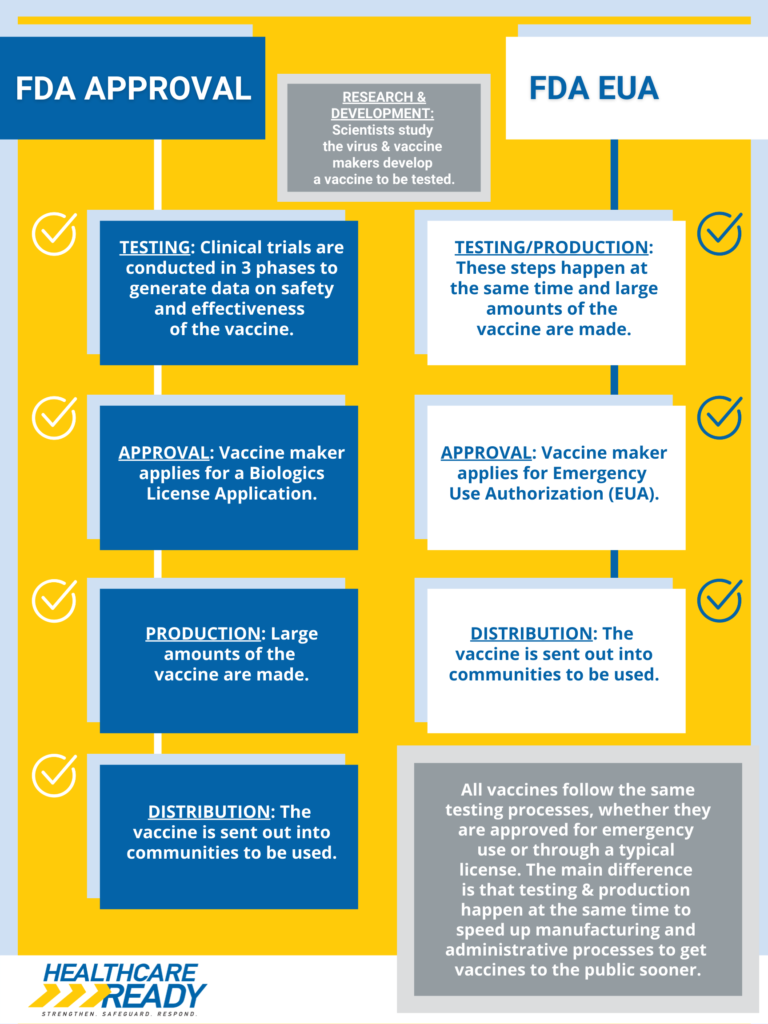
Healthcare Ready Infographic Fda Approval Vs Fda Emergency Use Under section 564 of the federal food, drug, and cosmetic act (fd&c act), when the secretary of hhs declares that an emergency use authorization is appropriate, fda may authorize unapproved. Expanded authorization allows more americans to receive a booster dose to help preserve a high level of protection against covid 19 pfizer inc. (nyse: pfe) and biontech se (nasdaq: bntx) today announced that the u.s. food and drug administration (fda) has expanded the emergency use authorization (eua) of a booster dose of the pfizer biontech covid 19 vaccine to include individuals 18 years of.

Comments are closed.