Fda Approves At Home Covid 19 Test

Fda Approves At Home Covid 19 Test Cbs Chicago The table below is updated regularly and lists fda authorized at home otc covid 19 diagnostic tests, including information on expiration dates, who can use the test, links to home use instructions. The announcement today of the first fully at home otc covid 19 diagnostic test follows last month’s authorization of the first prescription covid 19 test for home use and last week’s.

At Home Covid 19 Test Fda Approves Rapid 30 Minute Antigen Swab Kit Inquiries. lauren jei mccarthy. 240 702 3940. 888 info fda. the fda issued an emergency use authorization for the first covid 19 diagnostic test that allows for self collection and testing at home. Fda authorizes 2 rapid, at home coronavirus tests. april 1, 2021 4:37 am et. the newly authorized tests in the fight against covid 19 are abbott's binaxnow test and quidel's quickvue. In november 2023, the acon flowflex covid 19 antigen home test became the first at home rapid antigen test cleared through the food and drug administration (fda)’s traditional premarket review. The covid 19 at home test is a rapid chroma tographic immunoassay for the qualitative detection of the nucleocapsid protein of sars cov 2 present in anterior nasal swab samples. in a prospective clinical study, the covid 19 at home test showed a relative sensitivity of 95.3% (95% ci: 84.5 to 98.7%) and a relative specificity of 100% (95% ci: 95.

Fda Approves First Covid 19 Test Kit For Home Use вђ Metro Us In november 2023, the acon flowflex covid 19 antigen home test became the first at home rapid antigen test cleared through the food and drug administration (fda)’s traditional premarket review. The covid 19 at home test is a rapid chroma tographic immunoassay for the qualitative detection of the nucleocapsid protein of sars cov 2 present in anterior nasal swab samples. in a prospective clinical study, the covid 19 at home test showed a relative sensitivity of 95.3% (95% ci: 84.5 to 98.7%) and a relative specificity of 100% (95% ci: 95. Amazon. $ 42.99. target. flowflex’s home testing kit comes with five tests. after the first test, the brand recommends testing two more times at least 48 hours apart. the kit comes with. Fda lists all over the counter covid 19 tests authorized for home use. feb 23, 2022 02:46 pm. the food and drug administration yesterday listed all over the counter covid 19 diagnostic tests authorized for home use, including links to home use instructions for each test. novel coronavirus (sars cov 2 covid 19).
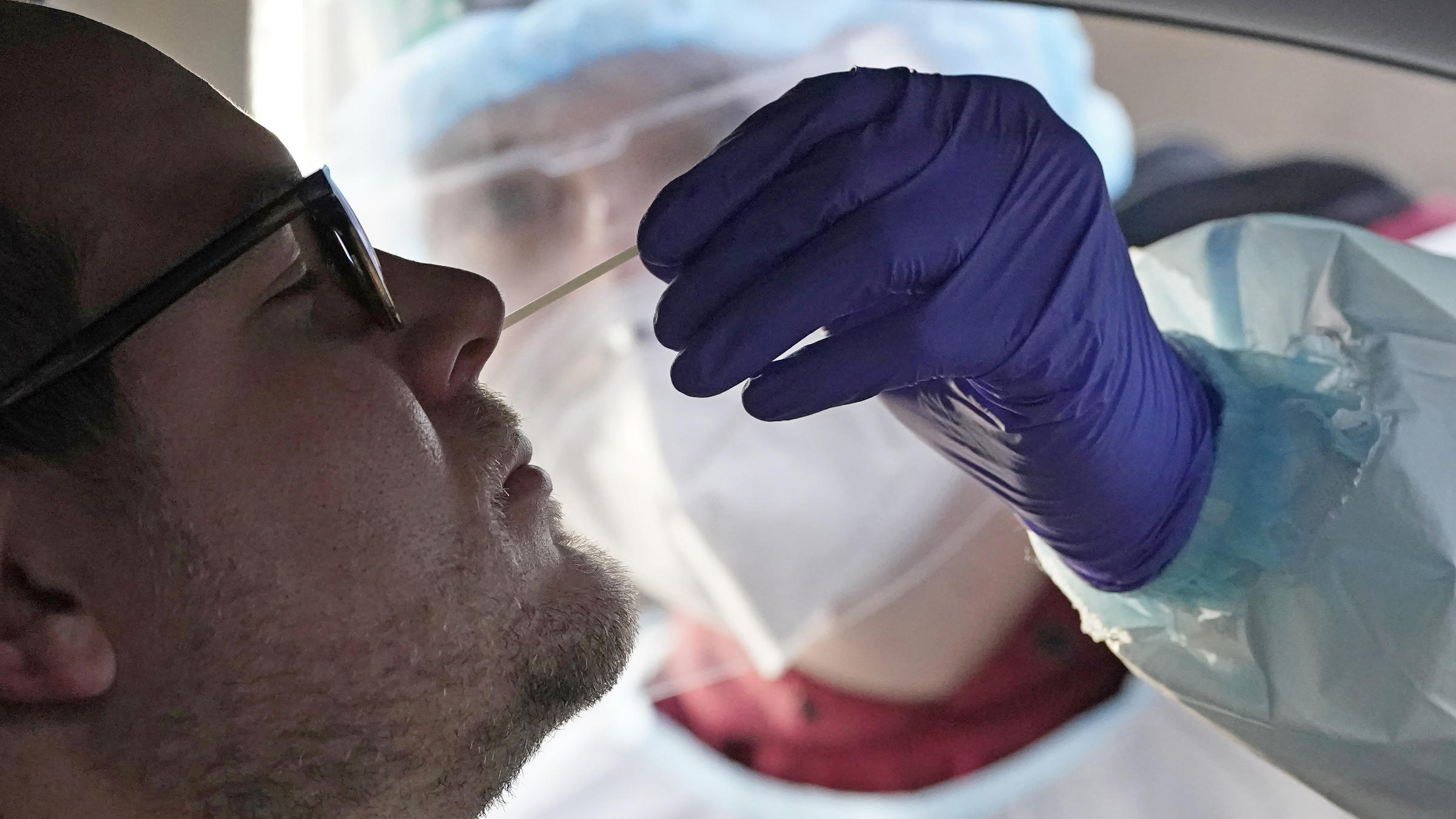
Fda Approves 1st At Home Coronavirus Test Wjct News Amazon. $ 42.99. target. flowflex’s home testing kit comes with five tests. after the first test, the brand recommends testing two more times at least 48 hours apart. the kit comes with. Fda lists all over the counter covid 19 tests authorized for home use. feb 23, 2022 02:46 pm. the food and drug administration yesterday listed all over the counter covid 19 diagnostic tests authorized for home use, including links to home use instructions for each test. novel coronavirus (sars cov 2 covid 19).

Comments are closed.