Exenatide Once Weekly Over 2 Years As A Potential Disea
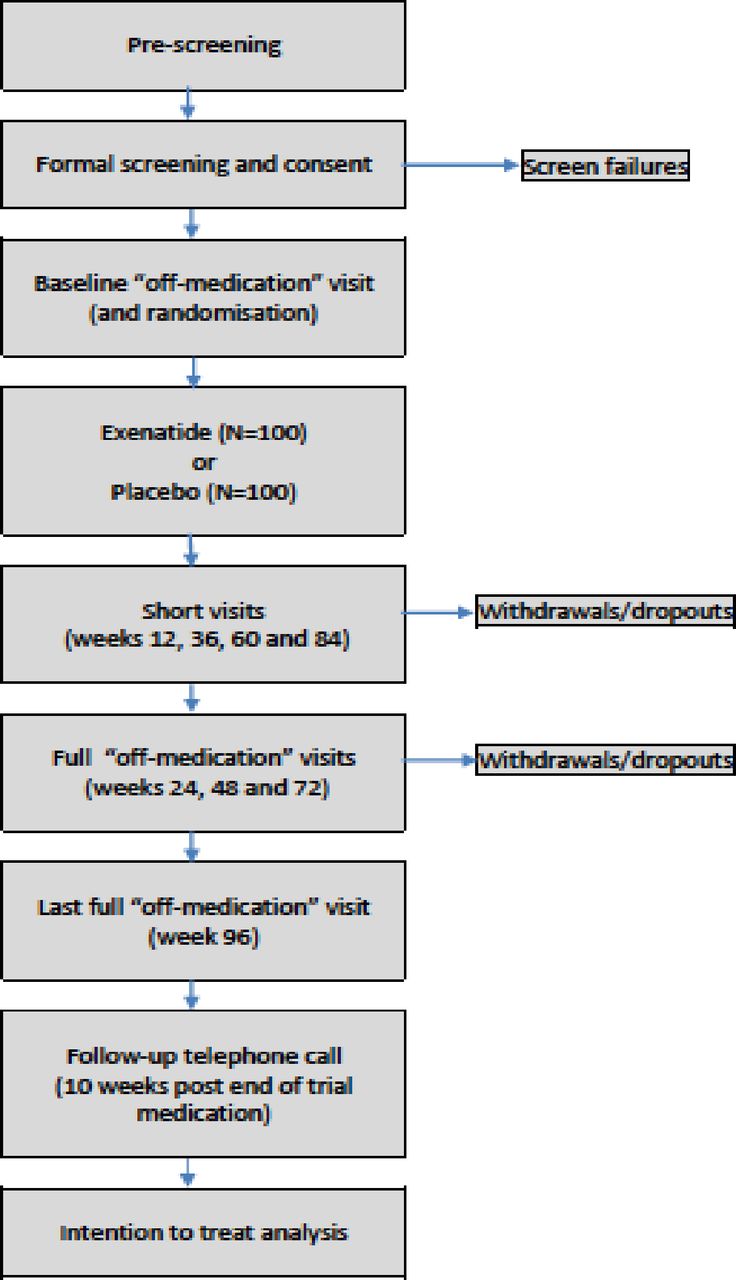
Exenatide Once Weekly Over 2 Years As A Potential Disea The glucagon like peptide 1 receptor agonist exenatide has been associated in single centre studies with reduced motor deterioration over 1 year. the aim of this multicentre uk trial is to confirm whether these previous positive results are maintained in a larger number of participants over 2 years and if effects accumulate with prolonged drug. To investigate the potential effects of exenatide in patients with pd, an investigator initiated pilot trial was undertaken. 13 this open label, parallel group, randomised controlled trial evaluated the tolerability of exenatide (byetta 10 μg two times per day) in 45 patients with moderately severe pd (hoehn and yahr stage of less than 2.5.

Pdf Exenatide Once Weekly Over 2 Years As A Potential Methods and analysis this is a phase 3, multicentre, double blind, randomised, placebo controlled trial of exenatide at a dose of 2 mg weekly in 200 participants with mild to moderate pd. Exenatide once weekly over 2 years as a potential disease modifying treatment for parkinson's disease: protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: the 'exenatide pd3' study. bmj open. 2021 may 28;11(5):e047993. pubmed. recommends. please login to recommend the paper. comments. This multicentre uk trial is to confirm whether these previous positive results are maintained in a larger number of participants over 2 years and if effects accumulate with prolonged drug exposure of exenatide. introduction parkinson’s disease (pd) is a common neurodegenerative disorder with substantial morbidity. no disease modifying treatments currently exist. the glucagon like peptide 1. Exenatide once weekly over 2 years as a potential disease modifying treatment for parkinson's disease: protocol for a multi centre, randomised, double blind, parallel group, placebo controlled, phase 3 trial, the 'exenatide pd3' study. lookup nu author(s): dr silvia del din orcid, professor alison yarnall, professor lynn rochester orcid. downloads.

Thuá C î Exenatideî Cã Ng Dá Ng Chá ä á Nh Vã Læ U ã Khi Dã Ng Vinmec This multicentre uk trial is to confirm whether these previous positive results are maintained in a larger number of participants over 2 years and if effects accumulate with prolonged drug exposure of exenatide. introduction parkinson’s disease (pd) is a common neurodegenerative disorder with substantial morbidity. no disease modifying treatments currently exist. the glucagon like peptide 1. Exenatide once weekly over 2 years as a potential disease modifying treatment for parkinson's disease: protocol for a multi centre, randomised, double blind, parallel group, placebo controlled, phase 3 trial, the 'exenatide pd3' study. lookup nu author(s): dr silvia del din orcid, professor alison yarnall, professor lynn rochester orcid. downloads. Type: article title: exenatide once weekly over 2 years as a potential disease modifying treatment for parkinson's disease: protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: the 'exenatide pd3' study. Exenatide once weekly over 2 years as a potential disease modifying treatment for parkinson’s disease (exenatide pd3).

New Formulation Of Once Weekly Exenatide Available For T2d Type: article title: exenatide once weekly over 2 years as a potential disease modifying treatment for parkinson's disease: protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: the 'exenatide pd3' study. Exenatide once weekly over 2 years as a potential disease modifying treatment for parkinson’s disease (exenatide pd3).

Comments are closed.