Endothermic And Exothermic Chemical Reactions
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Endothermic Reactions And Exothermic Reaction Chemical processes are labeled as exothermic or endothermic based on whether they give off or absorb energy, respectively. 7.3: exothermic and endothermic reactions is shared under a cc by nc sa 3.0 license and was authored, remixed, and or curated by libretexts. atoms are held together by a certain amount of energy called bond energy. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy ( Δh). other chemical reactions release energy in the form of heat, light, or sound. these are exothermic reactions. exothermic reactions may occur spontaneously and result in higher randomness or entropy (Δs > 0) of the system.

Endothermic And Exothermic Reaction Diagram An example of an exothermic reaction is the chemical reaction between sodium and chlorine, producing a bright yellow light and a great amount of heat energy. endothermic reactions the endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. in an exothermic reaction, heat is released (considered a product) and the energy of the system decreases (Δ h is negative). a chemical reaction is exothermic if heat is released by the system into the surroundings. An endothermic reaction feels cold because it absorbs heat from its surroundings. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. because heat is absorbed, endothermic reactions feel cold. Exothermic reactions are chemical reactions close chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. which release.
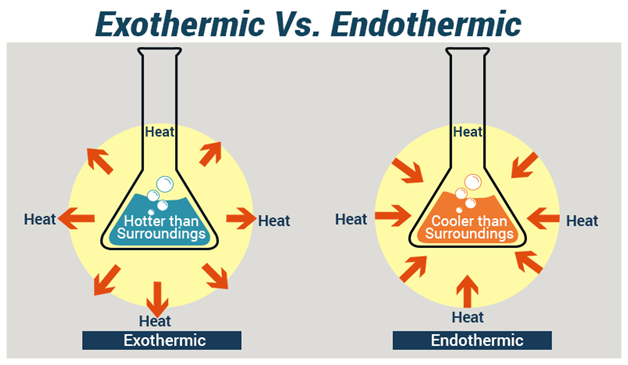
Difference Between Endothermic And Exothermic Reactions Chemistry An endothermic reaction feels cold because it absorbs heat from its surroundings. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. because heat is absorbed, endothermic reactions feel cold. Exothermic reactions are chemical reactions close chemical reaction when chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. which release. Q1. match the following terms to their definitions. exothermic chemical reaction . energy is transferred from the reactants to the surroundings. endothermic chemical reaction . energy from the surroundings is transferred to the products. dissipate . energy is transferred (and lost) as 'heat' to the environment. q2. Endothermic vs. exothermic reactions. chemistry in context may 31, 2024. exothermic and endothermic reactions high school energy from acs pressroom on vimeo. learn more about endothermic and exothermic reactions. more from this series. mountaintop mining. real talk: dr. doezema.

The Difference Between Endothermic And Exothermic Reactions Q1. match the following terms to their definitions. exothermic chemical reaction . energy is transferred from the reactants to the surroundings. endothermic chemical reaction . energy from the surroundings is transferred to the products. dissipate . energy is transferred (and lost) as 'heat' to the environment. q2. Endothermic vs. exothermic reactions. chemistry in context may 31, 2024. exothermic and endothermic reactions high school energy from acs pressroom on vimeo. learn more about endothermic and exothermic reactions. more from this series. mountaintop mining. real talk: dr. doezema.

Exothermic And Endothermic Reactions Key Differences

Comments are closed.