Complete The Table Below By Deciding Whether Precipitate Forms When Aqueous Solutions Of The Followi
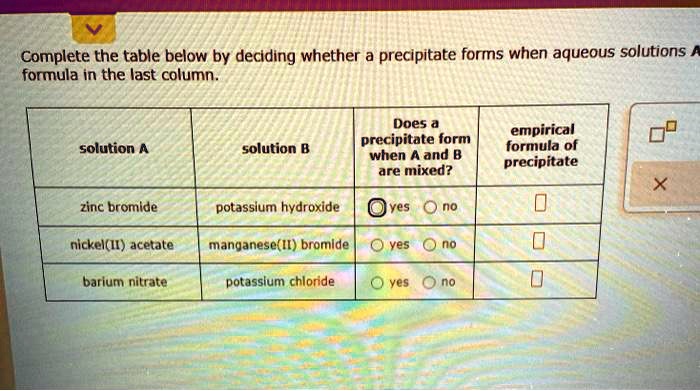
Solved Complete The Table Below By Deciding Whether A Precipitateођ Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. if a precipitate will form, enter its empirical formula in the last column. solution a solution b does a precipitate form when a and b are mixed empirical formula of precipitate manganese ii chloride zinc sulfate yes no empirical formula. Step 1. a) potassium sulfide and iron (ii) nitrate. according to solubility rules, all nitrates are soluble. s complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. if a precipitate will form, enter its empirical formula in the last column.
Solved Complete The Table Below By Deciding Whether A Precipitateо Which of the following options correctly describe the steps required to decide whether or not a precipitate forms when two aqueous solutions are mixed? select all that apply. any product that is insoluble in water will be a precipitate. Figure 4.2.1 4.2. 1: a precipitate of pbi 2 forms when solutions containing pb 2 and i − are mixed. (credit: der kreole wikimedia commons) the solubility guidelines in table 4.2.1 4.2. 1 may be used to predict whether a precipitation reaction will occur when solutions of soluble ionic compounds are mixed together. Hence co(oh) 2 will precipitate according to the following net ionic equation: \(co^{2 }(aq) 2oh^ (aq) \rightarrow co(oh) 2(s)\) a when aqueous solutions of strontium bromide and aluminum nitrate are mixed, we initially obtain a solution that contains sr 2 , br −, al 3 , and no 3 − ions. the two possible products from an exchange. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. if a precipitate will form, enter its empirical formula in the last column. solution a barium bromide potassium sulfide potassium hydroxide solution b potassium acetate zinc sulfate magnesium nitrate does a precipitate form when a and b are mixed? yes yes yes no no no empirical formula of.
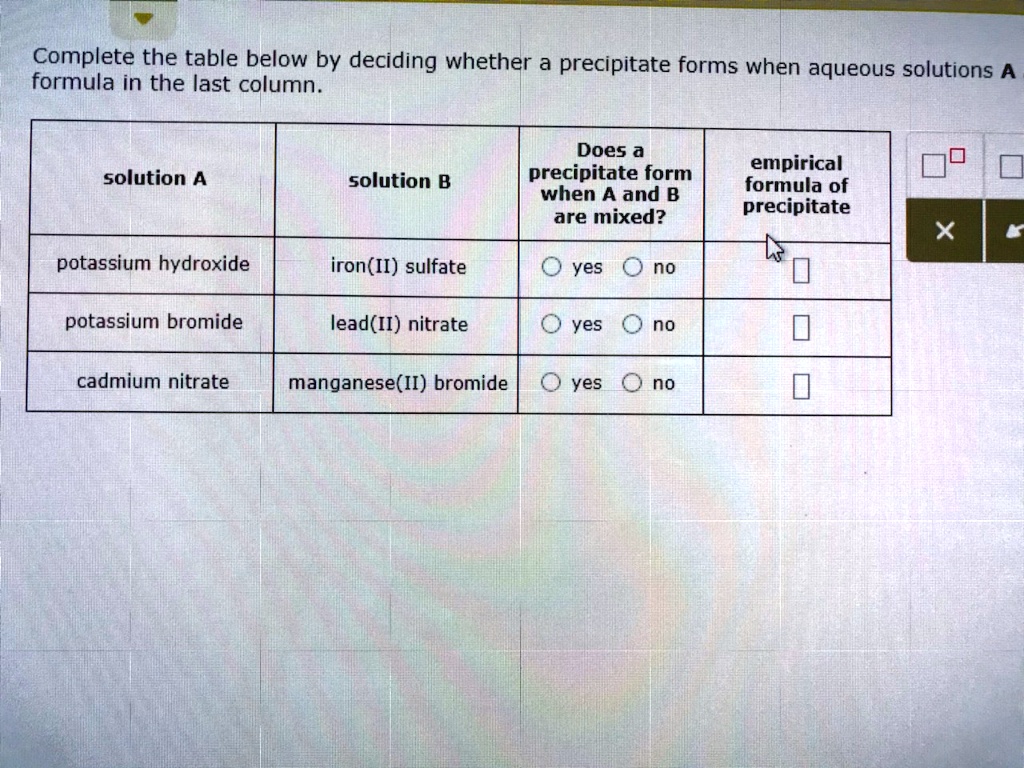
Solved Complete The Table Below By Deciding Whether A Precipitateођ Hence co(oh) 2 will precipitate according to the following net ionic equation: \(co^{2 }(aq) 2oh^ (aq) \rightarrow co(oh) 2(s)\) a when aqueous solutions of strontium bromide and aluminum nitrate are mixed, we initially obtain a solution that contains sr 2 , br −, al 3 , and no 3 − ions. the two possible products from an exchange. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. if a precipitate will form, enter its empirical formula in the last column. solution a barium bromide potassium sulfide potassium hydroxide solution b potassium acetate zinc sulfate magnesium nitrate does a precipitate form when a and b are mixed? yes yes yes no no no empirical formula of. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. if a precipitate will form, enter its empirical formula in the last column. solution a solution b does a precipitate form when a and b are mixed? empirical formula of precipitate cadmium nitrate iron(ii) chloride yes no sodium chloride barium nitrate yes no lead(ii) nitrate potassium. Video answer: answer: 1 reaction: 2, a g n, o 3 plus n, a 2 s; 22 n, a n o 3 plus a g 2 s, o n, h, 4, n, o 3 plus n, a c h, 3 c,… complete the table below by deciding whether precipitate forms when aqueous solutions and b are mixed if a precipitate will form, enter its empirical formula in the last column.

Solved Predicting Precipltotion Complete The Table Below By Deciding Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. if a precipitate will form, enter its empirical formula in the last column. solution a solution b does a precipitate form when a and b are mixed? empirical formula of precipitate cadmium nitrate iron(ii) chloride yes no sodium chloride barium nitrate yes no lead(ii) nitrate potassium. Video answer: answer: 1 reaction: 2, a g n, o 3 plus n, a 2 s; 22 n, a n o 3 plus a g 2 s, o n, h, 4, n, o 3 plus n, a c h, 3 c,… complete the table below by deciding whether precipitate forms when aqueous solutions and b are mixed if a precipitate will form, enter its empirical formula in the last column.
Solved Complete The Table Below By Deciding Whether A Precipitateо

Comments are closed.