Cdisc Sdtm And Adam For Survival Data Ppt
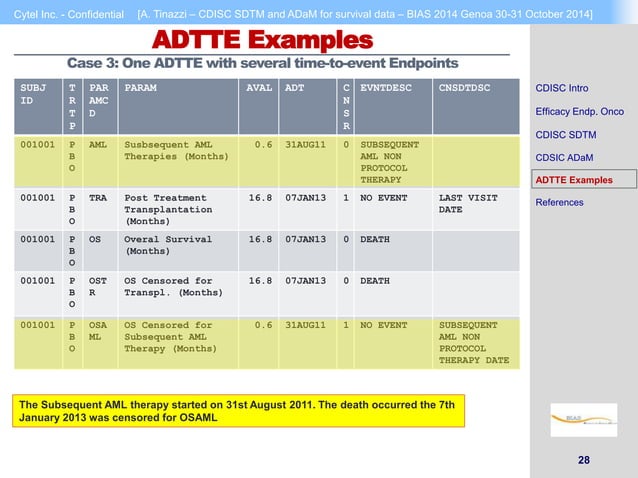
Cdisc Sdtm And Adam For Survival Data Ppt 3. cytel inc. confidential [a. tinazzi – cdisc sdtm and adam for survival data – bias 2014 genoa 30 31 october 2014] 3 cdisc intro cdisc intro efficacy endp. . onco cdisc sdtm cdsic adam adtte examples references cdisc is a global, open, multidisciplinary, non profit organization that has established standards to support the acquisition, exchange, submission and archive of clinical. Efficacy adams in oncology – step by step (dataset by dataset) woodcliff lake, usa simon lin, eisai inc., woodcliff lake, usaabstractthe cdisc adam team has designed the analysis data model (adam) standards to support submission to regulatory agencies such as the u.s. food and drug administratio. (fda) and japan’s pharmaceuticals and.

Cdisc Sdtm And Adam For Survival Data Ppt This paper utilizes the oncology adam datasets, adintdt and adtte, to create a list of censored subjects. the list provides information such as disposition date and status from treatment or study, source data of last known alive date, and other key study related dates. the list can assist the sites in following up with the currently censored. 1. introduction. survival analysis is a class of statistical methods for studying the occurrence and timing of events. describing the timeline over which key disease related events appear is an important feature of disease characterization. in many clinical studies, an outcome of interest is the time to an event. Adam defines dataset and metadata standards that support: traceability among analysis results, analysis data, and data represented in the study data tabulation model (sdtm). adam is one of the required standards for data submission to fda (u.s.) and pmda (japan). details on the requirements for fda are specified in the fda’s data standards. 1 of 26. download now. cdisc sdtm domain presentation. 1. combined data interchange standard consortium (cdisc) standard data tabulation model presented by: ankur sharma biostatistical programmer parexel international, baltimore, md, usa. 2. definitions cdisc : clinical data interchange standard consortium sdtm : standard data tabulation model.

Comments are closed.