Cdisc Clinical Research Glossary Version 5 0

Cdisc Clinical Research Glossary Version 5 0 The glossary effort was launched in 2002 to support other cdisc standards by clarifying and disambiguating key concepts in clinical research. over time, the volunteers who serve on the glossary team have enhanced its scope and utility by:. Person employed by a sponsor or by a contract research organization acting on a sponsor's behalf, who monitors the progress of investigator sites participating in a clinical study. at some sites (primarily in academic settings), clinical research coordinators are called cras. clinical research associate: clinical research coordinator (crc) c51811.
Cdisc Clinical Research Glossary Cdisc terminology is published by the us national cancer institute’s enterprise vocabulary services (nci evs) as a set of terminology products that support the use of and harmonization among the cdisc foundational standards. these products include protocol, send, cdash, sdtm, adam, define xml, and glossary. cdisc terminology consists of. Cdisc glossary v18.0 contains 9 new terms, 45 changes to existing content, and many new, or changes to, existing “clusters” of related terms that, when their definitions are read together, help sharpen the semantic distinctions and optimize effective communication. glossary v18.0 can also be found in excel, text, odm.xml, pdf, html, and owl. The purpose of the cdisc glossary is to serve the community of clinical researchers by selecting and defining terms pertaining to clinical research, particularly eclinical investigations, sponsored by the pharmaceutical industry or a federal agency. additional terminology will be developed on an ongoing basis. This organization, the clinical data interchange standards consortium (cdisc) is an open, non profit organization that develops and supports global data standards to improve the quality and interoperability of data in medical research and healthcare. cdisc partners with nci enterprise vocabulary services (evs) to develop and support controlled.

Cdisc Clinical Research Glossary The purpose of the cdisc glossary is to serve the community of clinical researchers by selecting and defining terms pertaining to clinical research, particularly eclinical investigations, sponsored by the pharmaceutical industry or a federal agency. additional terminology will be developed on an ongoing basis. This organization, the clinical data interchange standards consortium (cdisc) is an open, non profit organization that develops and supports global data standards to improve the quality and interoperability of data in medical research and healthcare. cdisc partners with nci enterprise vocabulary services (evs) to develop and support controlled. Cdisc term for a proposed uniform cdisc standard intended to address the full life cycle of a clinical trial including protocol representation, capture of source data, submission and archiving using a set of fully integrated and consistent models, terms and controlled vocabularies derived from the current set of cdisc standards. 2) a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health related biomedical or behavioral outcomes. (1. modified from ich e6 glossary, directive 2001 20 ec. 2.

Cdisc Clinical Research Glossary Pdf Clinical Trial Institutional Cdisc term for a proposed uniform cdisc standard intended to address the full life cycle of a clinical trial including protocol representation, capture of source data, submission and archiving using a set of fully integrated and consistent models, terms and controlled vocabularies derived from the current set of cdisc standards. 2) a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health related biomedical or behavioral outcomes. (1. modified from ich e6 glossary, directive 2001 20 ec. 2.
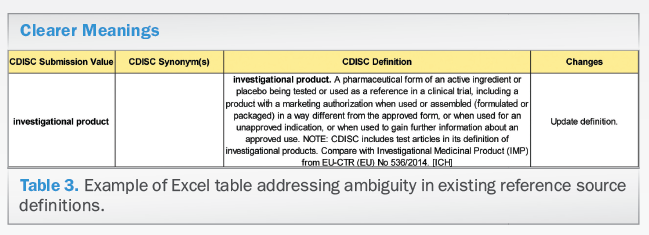
Cdisc Glossary Of Clinical Research Terminology

Comments are closed.