Cdisc Clinical Research Glossary Glossary Terms

Cdisc Clinical Research Glossary Glossary Terms The glossary team has worked with nci evs to link glossary terms with controlled terminology concepts. each year, the team updates 50 100 existing terms, reviews new guidances and current topics for terms, and updates acronyms abbreviations initials, if needed. in addition, authoritative sources for definitions are provided as a tab in the. Cdisc terminology is the set of cdisc developed or cdisc adopted standard expressions (values) used with data items within the foundational standards and therapeutic area user guides. cdisc terminology provides context, content, and meaning to clinical research data and provides a consistent semantic layer across all operational contexts, enabling interoperability of the cdisc standards.
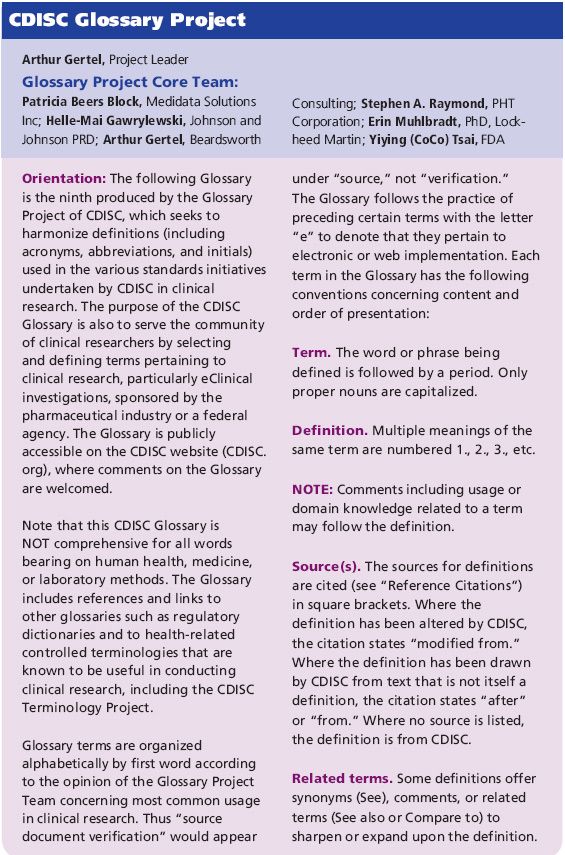
Cdisc Clinical Research Glossary Cdisc glossary v18.0 contains 9 new terms, 45 changes to existing content, and many new, or changes to, existing “clusters” of related terms that, when their definitions are read together, help sharpen the semantic distinctions and optimize effective communication. glossary v18.0 can also be found in excel, text, odm.xml, pdf, html, and owl. A clinical trial which fulfills all of the following conditions: (a) the investigational medicinal products, excluding placebos, are authorized; (b) according to the protocol of the clinical trial, (i) the investigational medicinal products are used in accordance with the terms of the marketing authorization; or (ii) the use of the investigational medicinal products is evidence based and. The purpose of the cdisc glossary is to serve the community of clinical researchers by selecting and defining terms pertaining to clinical research, particularly eclinical investigations, sponsored by the pharmaceutical industry or a federal agency. additional terminology will be developed on an ongoing basis. Cdisc leads the glossary project, which harmonizes definitions (including acronyms, abbreviations, and initials) that are in the various cdisc managed standards initiatives in clinical research. if you’re a clinical researcher, you can use the cdisc glossary to select and define terms related to clinical research that the pharmaceutical industry or a federal agency sponsors.

Comments are closed.