Cdisc And Cdisc Standards Egnyte
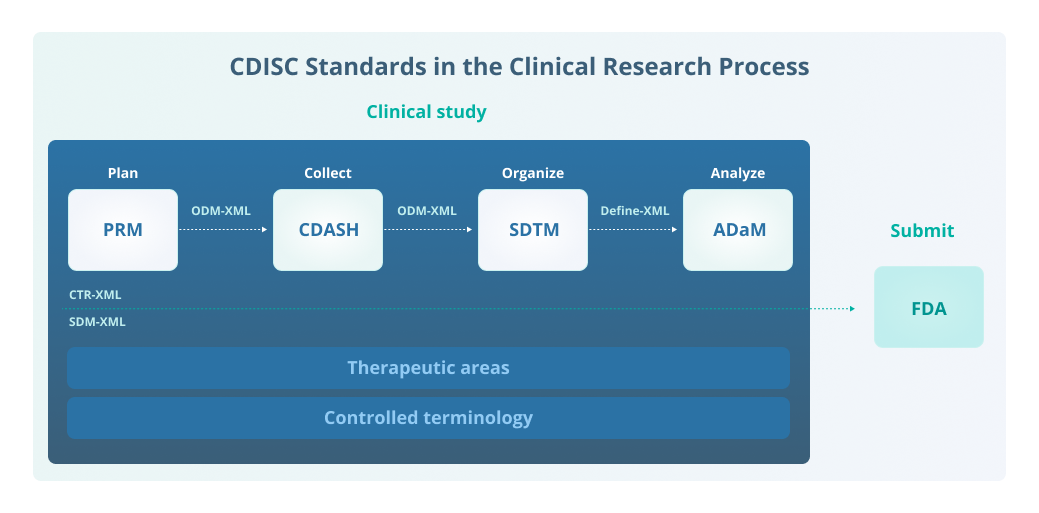
Cdisc And Cdisc Standards Egnyte The cdisc library is a central repository where cdisc metadata standards are developed, integrated, and accessed. it provides cdisc content standards in a machine readable format. by providing an api, the cdisc library facilitates the implementation of the standards with clinical trial software (e.g., a clinical trial management system or ctms). Cdisc foundational standards are the basis of the complete suite of standards, supporting clinical and non clinical research processes from end to end. foundational standards focus on the core principles for defining data standards and include models, domains and specifications for data representation.

Cdisc And Cdisc Standards Egnyte Clinical data management must follow a number of regulations, guidelines, and standards, including the following. clinical data interchange standards consortium (cdisc) adherence to cdisc standards is required by the united states food and drug administration (fda) and japan’s pharmaceuticals and medical devices agency (pmda). Clear impact. with hundreds of employees, volunteers, and member organizations around the world. cdisc foundational standards are the basis for the complete cdisc suite of standards, supporting the clinical and non clinical research process from protocol through data collection, data exchange, data management, data analysis and reporting. The clinical data interchange standards consortium (cdisc) is a standards developing organization (sdo) dealing with medical research data linked with healthcare,made to enable information system interoperability and to improve medical research and related areas of healthcare. the standards support medical research from protocol through. Cdisc sdtm consists of 2 parts, the underlying study data tabulation model and implementation guides (sdtm igs) that define how the sdtm should be used to represent some common data domains in human clinical trials. the core model provides a standardized set of variables, which are grouped into ’classes’.

Cdisc And Cdisc Standards Egnyte The clinical data interchange standards consortium (cdisc) is a standards developing organization (sdo) dealing with medical research data linked with healthcare,made to enable information system interoperability and to improve medical research and related areas of healthcare. the standards support medical research from protocol through. Cdisc sdtm consists of 2 parts, the underlying study data tabulation model and implementation guides (sdtm igs) that define how the sdtm should be used to represent some common data domains in human clinical trials. the core model provides a standardized set of variables, which are grouped into ’classes’. The clinical data interchange standards consortium (cdisc) encompasses a suite of standards across the clinical space. the study data tabulation model (sdtm) and analysis data model (adam) are probably the two standards most familiar to pharmasug attendees, but there are many others. this paper and presentation focus on the foundational. With the help of cdisc standards, the entire research community can maximize the value of data for more efficient and meaningful research that has invaluable impact on global health. cdisc is a 501(c)(3) global nonprofit charitable organization and is headquartered in austin, texas, with hundreds of employees, volunteers, and member.
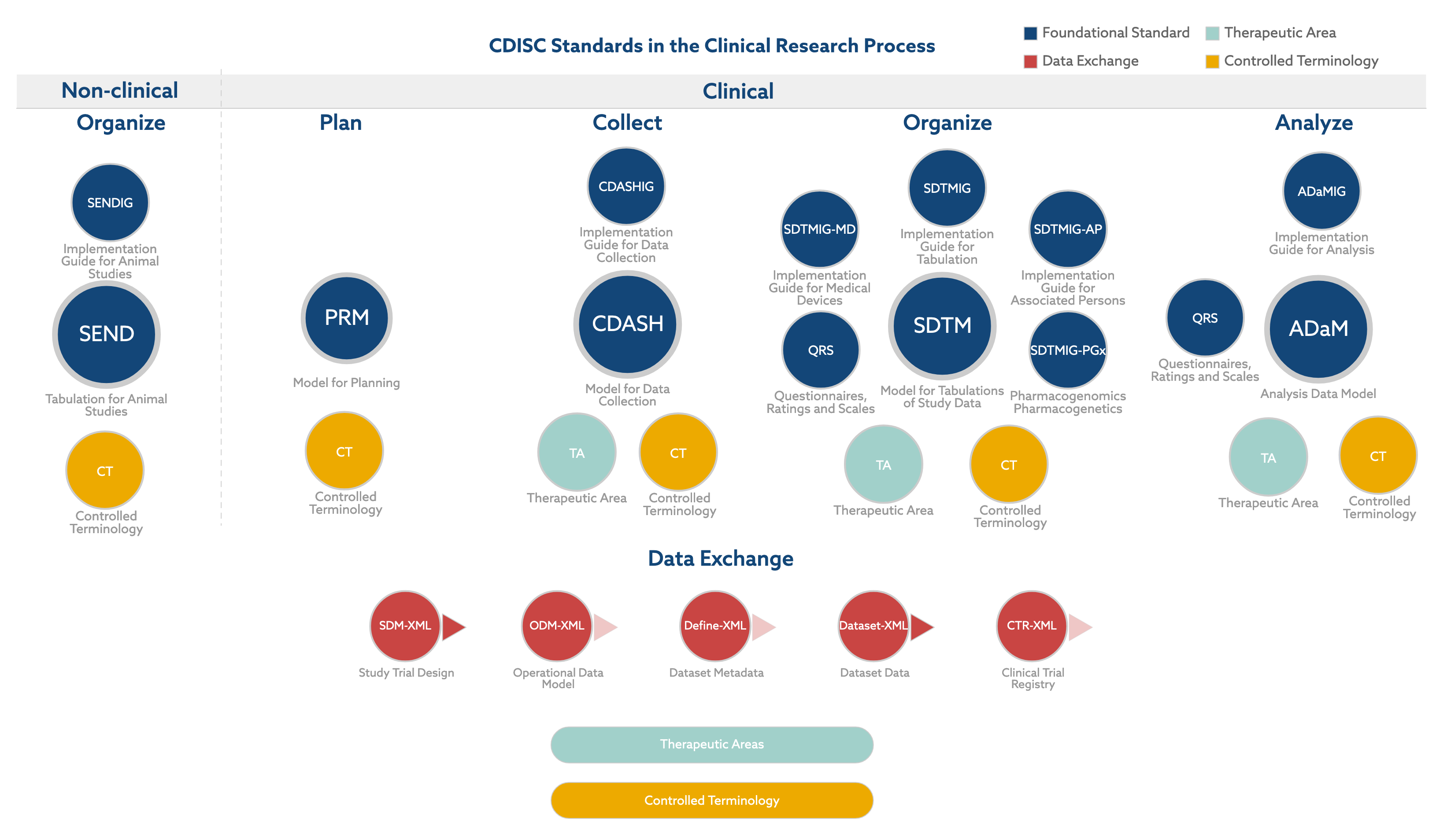
Standard Diagram Static Cdisc Vrogue Co The clinical data interchange standards consortium (cdisc) encompasses a suite of standards across the clinical space. the study data tabulation model (sdtm) and analysis data model (adam) are probably the two standards most familiar to pharmasug attendees, but there are many others. this paper and presentation focus on the foundational. With the help of cdisc standards, the entire research community can maximize the value of data for more efficient and meaningful research that has invaluable impact on global health. cdisc is a 501(c)(3) global nonprofit charitable organization and is headquartered in austin, texas, with hundreds of employees, volunteers, and member.

Comments are closed.