Bydureon Bcise Exenatide Extended Release Injectable Suspension Uses
Dailymed Bydureon Bcise Exenatide Injection Suspension Extended Bydureon bcise should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis. bydureon (exenatide for extended release injectable suspension), bydureon bcise, or byetta® (exenatide twice daily) should not be used concomitantly, as they contain the same medicinal ingredient and this could result in an overdose. Do not use if particulate matter is present or if discoloration is observed.< item> <item> <caption>•< caption>administer bydureon bcise immediately after the autoinjector is prepared as a subcutaneous injection in the abdomen, thigh, or upper arm region. advise patients to use a different injection site each week when injecting in the same.

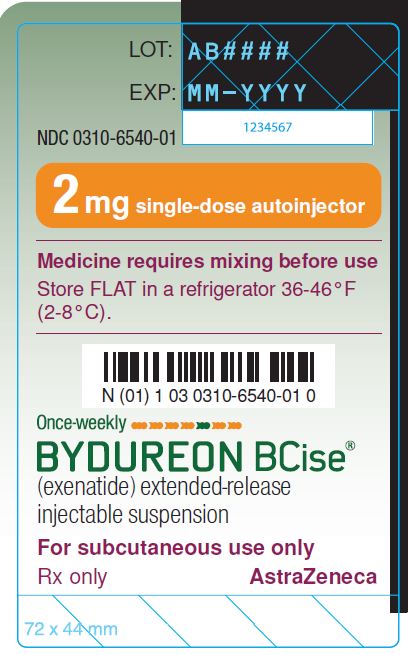
Bydureon Bcise Exenatide Extended Release Injectable Suspension Uses Extended release injectable suspension: 2 mg of exenatide per 0.85 ml suspension, in a pre filled single dose autoinjector. redispersion by mixing provides a white to off white, opaque 4 contraindications. bydureon bcise is contraindicated in patients with a: • personal or family history of medullary thyroid carcinoma (mtc) or in patients. Bydureon bcise is an extended release form of exenatide administered as an injection once every seven days. the dose can be administered at any time of day, with or without meals. follow your doctor's instructions. related similar drugs ozempic, rybelsus, metformin, trulicity, lantus, tresiba, victoza before taking this medicine. Common brand name(s): bydureon bcise common generic name(s): exenatide; exenatide extended release injectable suspension; exenatide microspheres pronunciation: by dur ee on b cise drug classes. Bydureon® exenatide for extended release injectable suspension 2 mg dose once weekly atc code: a10bj01 glucagon like peptide 1 (glp 1) analogues astrazeneca canada inc. 1004 middlegate road, suite 5000 mississauga, ontario l4y 1m4 astrazeneca.ca date of preparation: january 20, 2020 submission control no: 233114.

Dailymed Bydureon Bcise Exenatide Injection Suspension Extended Common brand name(s): bydureon bcise common generic name(s): exenatide; exenatide extended release injectable suspension; exenatide microspheres pronunciation: by dur ee on b cise drug classes. Bydureon® exenatide for extended release injectable suspension 2 mg dose once weekly atc code: a10bj01 glucagon like peptide 1 (glp 1) analogues astrazeneca canada inc. 1004 middlegate road, suite 5000 mississauga, ontario l4y 1m4 astrazeneca.ca date of preparation: january 20, 2020 submission control no: 233114. Wilmington, del., july 23, 2021 – astrazeneca’s bydureon bcise (exenatide extended release), once weekly injectable suspension has been approved in the us for the treatment of type 2 diabetes (t2d); to improve glycemic control in pediatric patients (10 to 17 years) as an adjunct to diet and exercise. Bydureon ® ([exenatide extended release] injectable suspension) [prescribing information]. wilmington, de: astrazeneca pharmaceuticals lp; 2020. data on file, re 5087, astrazeneca pharmaceuticals lp.
What Is Bydureon Bcise Goodrx Wilmington, del., july 23, 2021 – astrazeneca’s bydureon bcise (exenatide extended release), once weekly injectable suspension has been approved in the us for the treatment of type 2 diabetes (t2d); to improve glycemic control in pediatric patients (10 to 17 years) as an adjunct to diet and exercise. Bydureon ® ([exenatide extended release] injectable suspension) [prescribing information]. wilmington, de: astrazeneca pharmaceuticals lp; 2020. data on file, re 5087, astrazeneca pharmaceuticals lp.

Comments are closed.