Balancing Chemical Equations 2
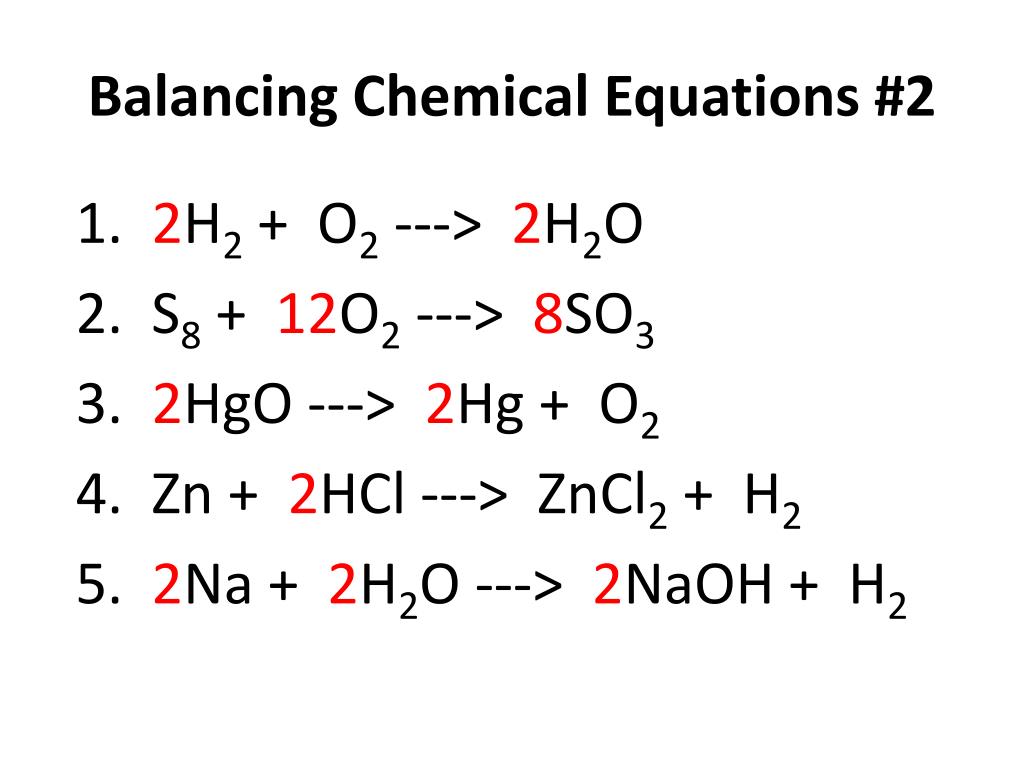
Ppt Balancing Chemical Equations 2 Powerpoint Presentation Free Practice by balancing a few of the equations below. if you get stuck, click the links to use our chemical equation balance calculator to see the balanced result and the four easy steps to get there: aluminium sodium hydroxide water = sodium aluminate hydrogen gas: al naoh h2o = naalo2 h2. This means that you will need to balance the carbon atoms first. 5. use a coefficient to balance the single carbon atom. add a coefficient to the single carbon atom on the right of the equation to balance it with the 3 carbon atoms on the left of the equation. c 3 h 8 o 2 > h 2 o 3 co 2.

Balancing Chemical Equations Summary The balanced chemical equation for the combustion of glucose in the laboratory (or in the brain) is as follows: c 6h 12o 6(s) 6o 2(g) → 6co 2(g) 6h 2o(l) construct a table showing how to interpret the information in this equation in terms of. a single molecule of glucose. moles of reactants and products. Instructions on balancing chemical equations: enter an equation of a chemical reaction and click 'balance'. the answer will appear below; always use the upper case for the first character in the element name and the lower case for the second character. examples: fe, au, co, br, c, o, n, f. compare: co cobalt and co carbon monoxide. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: al (s) o 2 (g) → al 2 o 3 (s) solution. the most complex formula is al 2 o 3 (s) and should be balanced first, while the simplest formula is al (s) and should be balanced last. Learn to balance chemical equations through interactive simulations and challenges on phet.
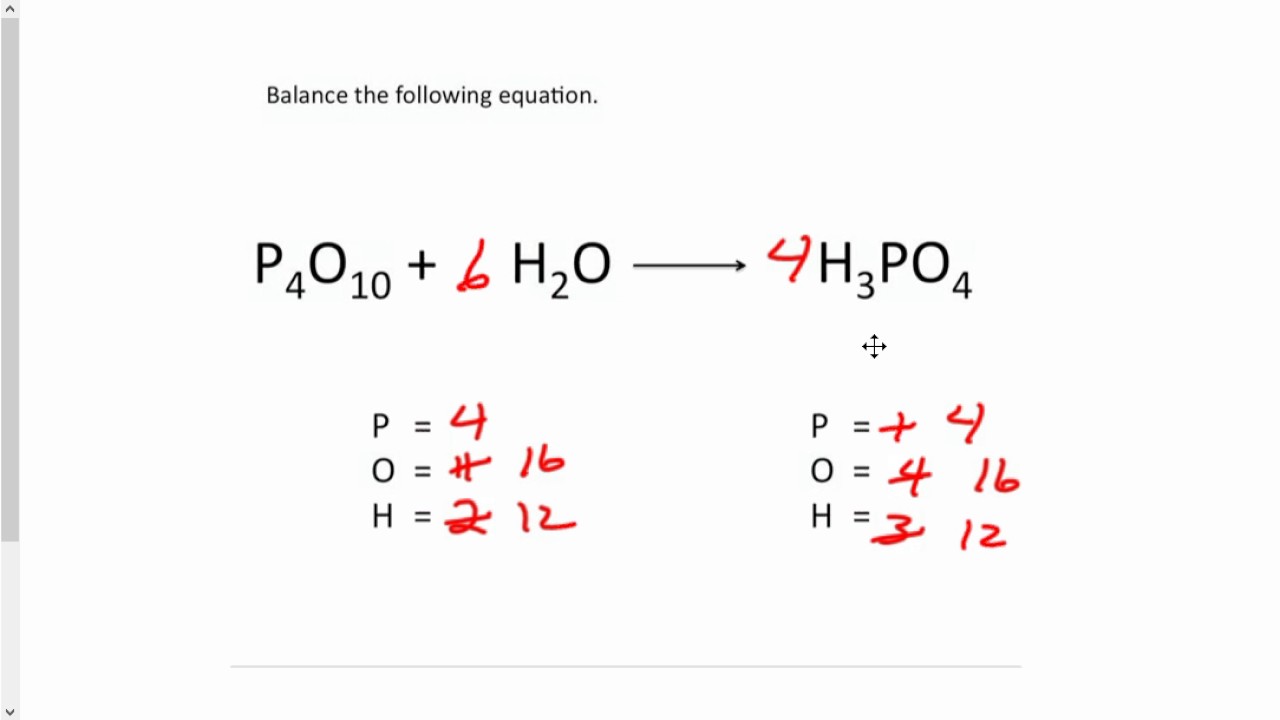
Balancing Chemical Equations 2 Youtube Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: al (s) o 2 (g) → al 2 o 3 (s) solution. the most complex formula is al 2 o 3 (s) and should be balanced first, while the simplest formula is al (s) and should be balanced last. Learn to balance chemical equations through interactive simulations and challenges on phet. Balancing equations. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. this is a requirement the equation must satisfy to be consistent with the law of conservation of matter. 2 × 2 = 4. (1 × 2) (2 × 1) = 4. 4 = 4, yes. a balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. consider as an example the decomposition of water to yield molecular hydrogen and oxygen. this process is represented.
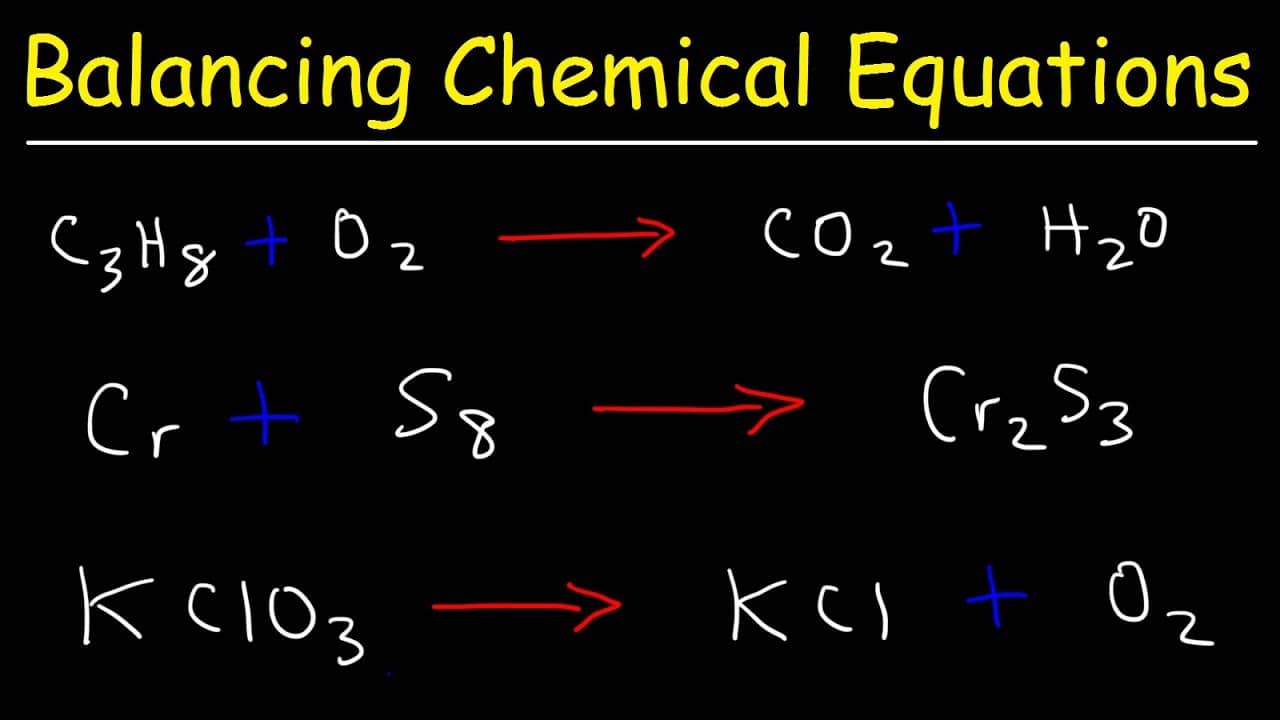
How To Balance Chemical Equations Best Examples Get Education Bee Balancing equations. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. this is a requirement the equation must satisfy to be consistent with the law of conservation of matter. 2 × 2 = 4. (1 × 2) (2 × 1) = 4. 4 = 4, yes. a balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. consider as an example the decomposition of water to yield molecular hydrogen and oxygen. this process is represented.
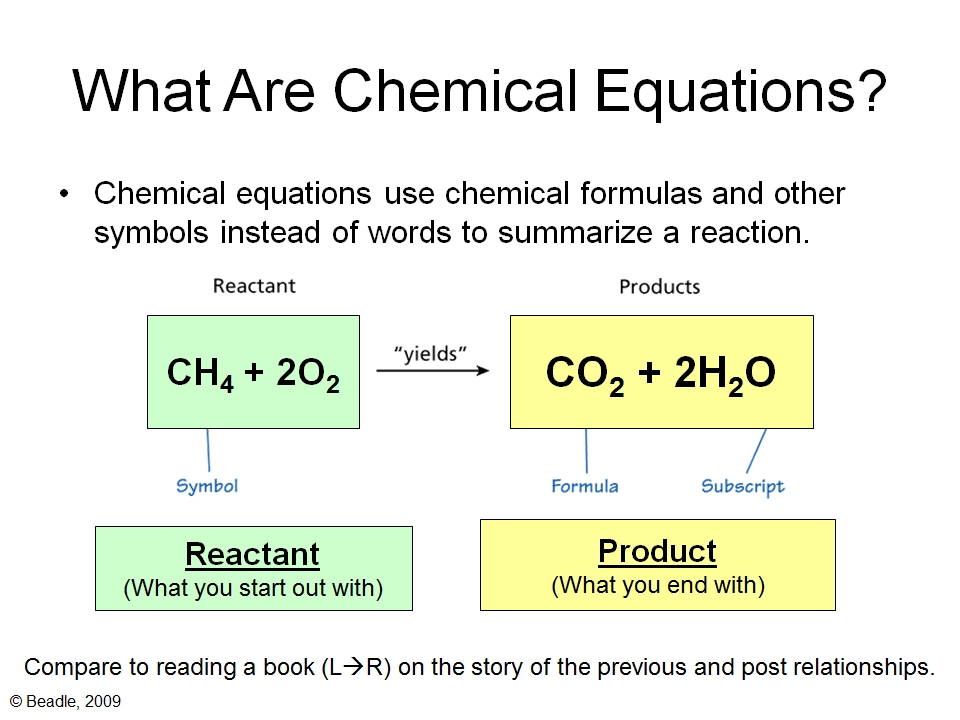
Balancing Chemical Equations Vista Heights 8th Grade Science

Comments are closed.