Balancing Both Sides Of An Equation

Balancing Both Sides Of An Equation Youtube In this video, you will learn how to keep both sides of an equation equal. we hope you are enjoying our large selection of engaging core & elective k 12 lear. The equation is now balanced, but we usually write equations with whole number coefficients. we can eliminate the fractional coefficient by multiplying all coefficients on both sides of the chemical equation by 2: . 2 c 8h 18(l) 25 o 2(g) 16 co 2(g) 18 h 2o(g) 5. check your work.

Balancing Both Sides Of An Equation This equation is not balanced because there are an unequal amount of h's, n's and o's on both sides of the equation. to balance the given equation, put a 3 in front of the hno 3 on the left hand side to get 3 n's on both sides:. In a balanced chemical equation, both the numbers of each type of atom and the total charge are the same on both sides. equations \(\ref{3.1.1}\) and \(\ref{3.1.2}\) are balanced chemical equations. what is different on each side of the equation is how the atoms are arranged to make molecules or ions. This means that you will need to balance the carbon atoms first. 5. use a coefficient to balance the single carbon atom. add a coefficient to the single carbon atom on the right of the equation to balance it with the 3 carbon atoms on the left of the equation. c 3 h 8 o 2 > h 2 o 3 co 2. Balancing for charge means you have the same net charge on both the reactant and product side of the equation. this isn't always zero! this isn't always zero! here's an example of how to balance the reaction between potassium permanganate and iodide ion in aqueous sulfuric acid to form potassium iodide and manganese(ii) sulfate.
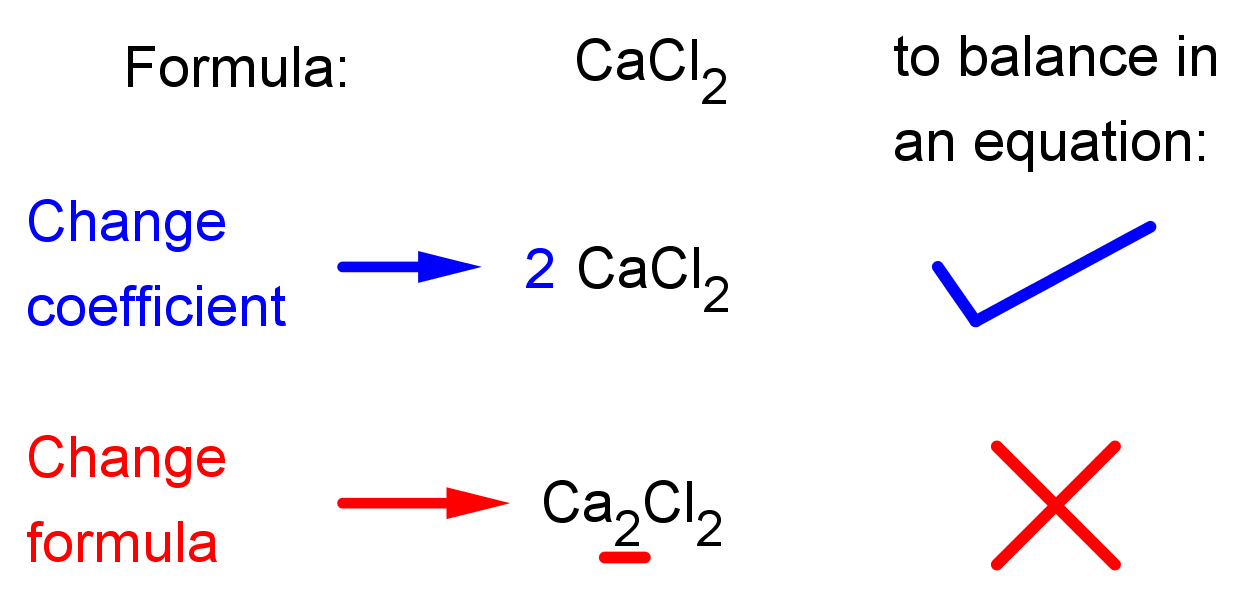
Master Balancing Chemical Equations Step By Step Guide Studypug This means that you will need to balance the carbon atoms first. 5. use a coefficient to balance the single carbon atom. add a coefficient to the single carbon atom on the right of the equation to balance it with the 3 carbon atoms on the left of the equation. c 3 h 8 o 2 > h 2 o 3 co 2. Balancing for charge means you have the same net charge on both the reactant and product side of the equation. this isn't always zero! this isn't always zero! here's an example of how to balance the reaction between potassium permanganate and iodide ion in aqueous sulfuric acid to form potassium iodide and manganese(ii) sulfate. ⚓️ strategies for balancing chemical equations. the most complex formula should usually be balanced first. the simplest formula should usually be balanced last. the least common multiple between two numbers may used to determine the coefficients. polyatomic ions (if present on both sides of the chemical equation) may be balanced as a unit. Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. consider as an example the reaction between one methane molecule (ch 4 ) and two diatomic oxygen molecules (o 2 ) to produce one carbon dioxide.

Comments are closed.