Balance Chemical Equations Solutions Examples Videos

How To Balance Chemical Equations Youtube Balance the following chemical equation: pb (no 3) 2 na 2 co 3 → pbco 3 nano 3. show video lesson. case 2: if the polyatomic ion is changed after the reaction then it would be necessary to consider each atom individually. example: balance the chemical equation. ba (oh) 2 h 3 po 4 → bahpo 4 h 2 o. solution:. A chemistry tutorial designed to help learn the basic principles of balancing chemical equations, along with examples and methods of balancing different chem.
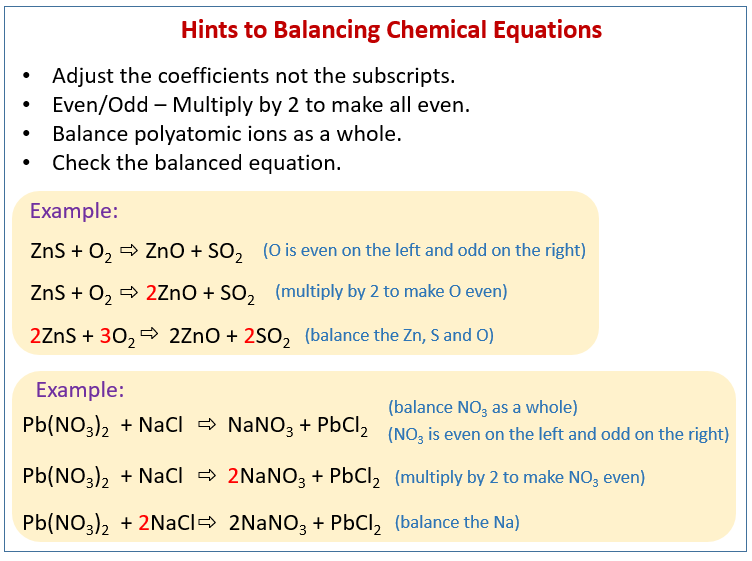
Balance Chemical Equations Solutions Examples Videos Solution: step 1: on the left side of the equation there are 2 oxygen atoms and on the right side of the equation there is one oxygen atoms. multiply k 2 o by the coefficient of 2 to balance the oxygen atoms. k o 2 → 2k 2 o. step 2: balance the k by placing the coefficient of 4 in front of k. 4k o 2 → 2k 2 o. How to balance chemical equations. we'll start out with examples that show the concepts behind balancing chemical equations. we will start with a word equat. This chemistry video shows you how to balance chemical equations especially if you come across a fraction or an equation with polyatomic ions. the goal is to. Example: balance the equation. ch 3 oh o 2 → h 2 o co 2. solution: step 1: using the cho technique, we start with carbon, one on each side, so carbon is balanced. there are four h on the left and two on the right, so we place the coefficient of 2 in front of the h 2 o on the right. ch 3 oh o 2 → 2h 2 o co 2.
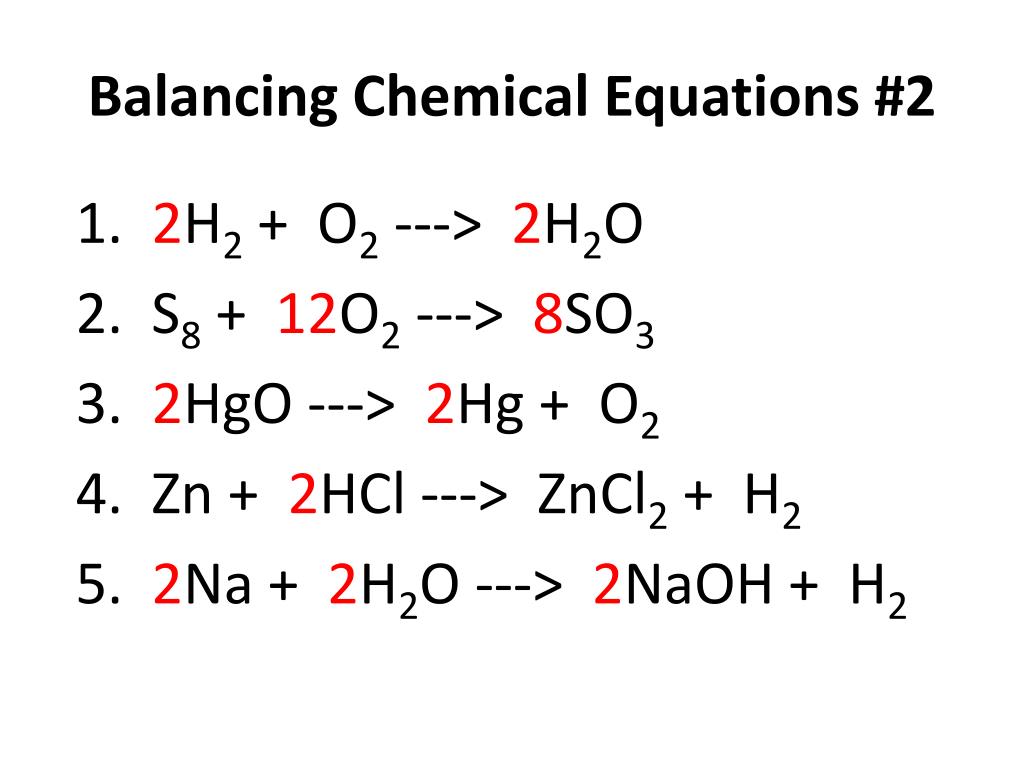
50 Examples Of Balanced Chemical Equations This chemistry video shows you how to balance chemical equations especially if you come across a fraction or an equation with polyatomic ions. the goal is to. Example: balance the equation. ch 3 oh o 2 → h 2 o co 2. solution: step 1: using the cho technique, we start with carbon, one on each side, so carbon is balanced. there are four h on the left and two on the right, so we place the coefficient of 2 in front of the h 2 o on the right. ch 3 oh o 2 → 2h 2 o co 2. A key step in balancing chemical equations is correctly identifying the formulas of the reactants and products. for example, consider the reaction between calcium oxide, cao(s), and h2o1l2 to form aqueous calcium hydroxide. (b) is it possible to balance the equation if you incorrectly identify the product as caoh1aq2, and if so, what is the. Co2 h2o → c6h12o6 o2. the first step to balancing chemical equations is to focus on elements that only appear once on each side of the equation. here, both carbon and hydrogen fit this requirement. so, we will start with carbon. there is only one atom of carbon on the left hand side, but six on the right hand side.

Comments are closed.