3 4 Chemical Equation Part 3 Tips And Tricks On Balancing Chemical Equation

Balancing Chemical Equations Summary Video balancing chemical equation demonstrated example 3: balance the chemical equation below. al(oh) 3(aq) li 2 co 3(aq) ——> lioh (aq) al 2 (co 3) 3(s) step 1: how do we start to balance a chemical equation? answer: same as before, first we write down the chemical equations on our paper and then we draw a line down the yield sign to. The first step in balancing a chemical equation is to identify your reactants and your products. remember, your reactants are on the left side of your equation. the products are on the right side. for this equation, our reactants are fe and o2. our products are fe2 and o 3.

Tips And Tricks For Balancing Chemical Equations Youtube This means that you will need to balance the carbon atoms first. 5. use a coefficient to balance the single carbon atom. add a coefficient to the single carbon atom on the right of the equation to balance it with the 3 carbon atoms on the left of the equation. c 3 h 8 o 2 > h 2 o 3 co 2. 4.3: videos balancing chemical equations is shared under a cc by 4.0 license and was authored, remixed, and or curated by libretexts. back to top 4.2: writing and balancing chemical equations. A balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. basically, this means there are the same numbers of each type of atoms on the left side of the equation as there are on the right side of the equation. it sounds like it should be simple to balance equations, but it's. This is a typical acid reaction. first, write the unbalanced chemical equation: kmno 4 ki h2so 4 → i 2 mnso 4. write down the oxidation numbers for each type of atom on both sides of the equation: left hand side: k = 1; mn = 7; o = 2; i = 0; h = 1; s = 6. right hand side: i = 0; mn = 2, s = 6; o = 2.
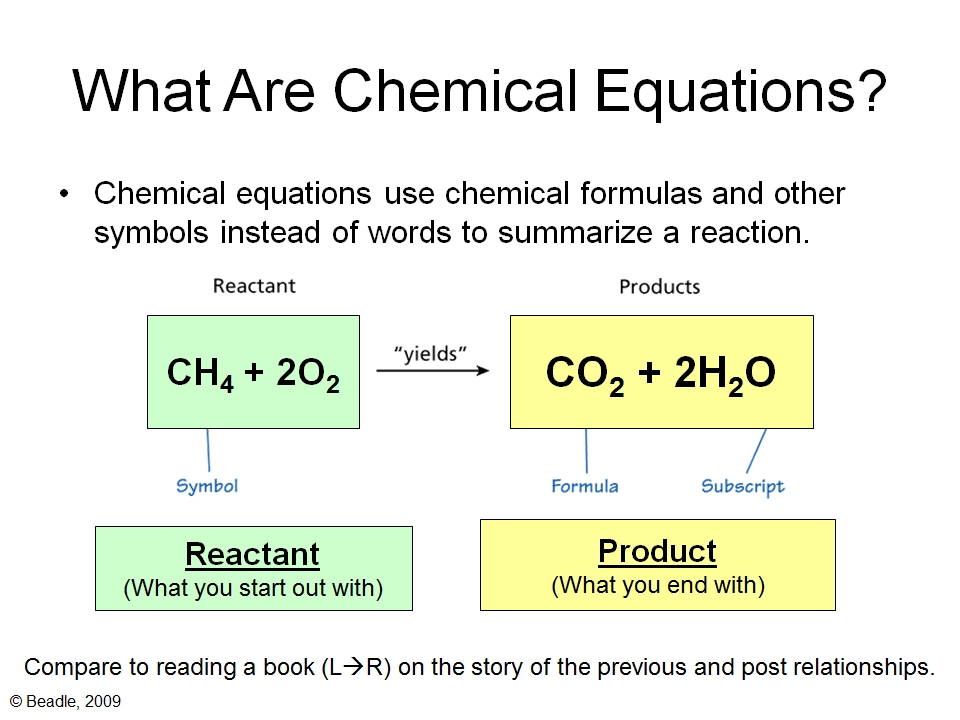
Balancing Chemical Equations Vista Heights 8th Grade Science A balanced chemical equation tells you the amounts of reactants and products needed to satisfy the law of conservation of mass. basically, this means there are the same numbers of each type of atoms on the left side of the equation as there are on the right side of the equation. it sounds like it should be simple to balance equations, but it's. This is a typical acid reaction. first, write the unbalanced chemical equation: kmno 4 ki h2so 4 → i 2 mnso 4. write down the oxidation numbers for each type of atom on both sides of the equation: left hand side: k = 1; mn = 7; o = 2; i = 0; h = 1; s = 6. right hand side: i = 0; mn = 2, s = 6; o = 2. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: al (s) o 2 (g) → al 2 o 3 (s) solution. the most complex formula is al 2 o 3 (s) and should be balanced first, while the simplest formula is al (s) and should be balanced last. One of the most bizarre reactions in chemistry is called the ugi reaction: r1c (=o)r2 r3 nh2 r4cooh r5nc s r4c (=o)n (r3)c (r1r2)c=onhr5 h2o (a) write out the balanced chemical equation for the ugi reaction, for the case where r = ch3ch2ch2ch2ch2ch2— (this is called the hexyl group) for all compounds. 491.

How To Do Balancing Equations Chemistry Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: al (s) o 2 (g) → al 2 o 3 (s) solution. the most complex formula is al 2 o 3 (s) and should be balanced first, while the simplest formula is al (s) and should be balanced last. One of the most bizarre reactions in chemistry is called the ugi reaction: r1c (=o)r2 r3 nh2 r4cooh r5nc s r4c (=o)n (r3)c (r1r2)c=onhr5 h2o (a) write out the balanced chemical equation for the ugi reaction, for the case where r = ch3ch2ch2ch2ch2ch2— (this is called the hexyl group) for all compounds. 491.

How To Balance Chemical Equations 11 Steps With Pictures

Comments are closed.