09 19 17 The Virtuous Cycle Biomarkers And Translational Research In Asthma Drug Development
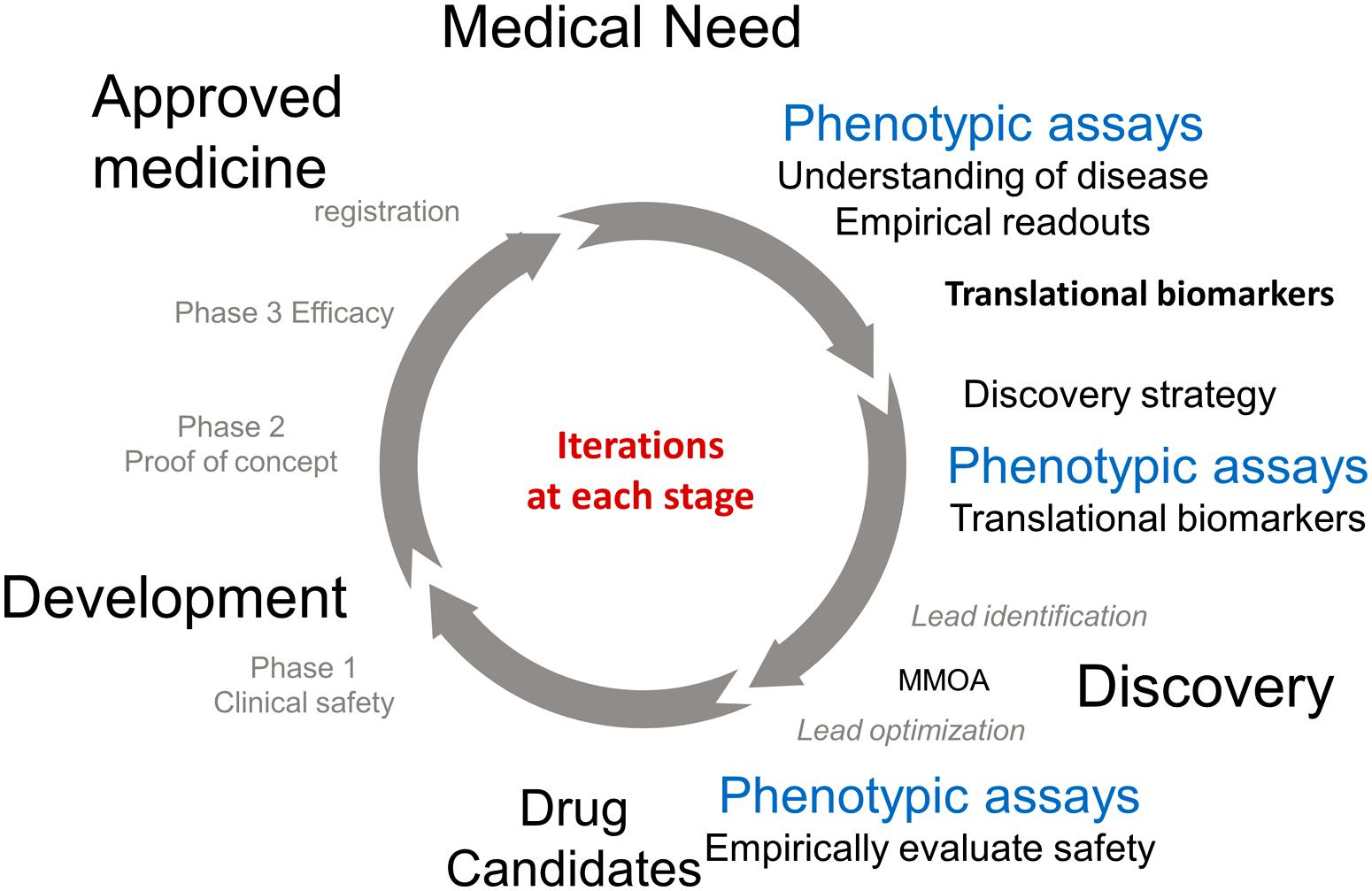
Frontiers The Value Of Translational Biomarkers To Phenotypic Assays Allergy and immunology teaching series presentation by joseph arron, md, phd, genentech, inc. A generic process of biomarker discovery and development begins with the analysis of a large number of biomarkers (analytes) and or tests in a small number of samples and culminates in the.
New Insights Into Asthma Drug Development As both drug development and biomarker development are extremely cost and time intensive, it might be challenging for a sponsor to develop both simultaneously. 16 the current regulatory landscape for biomarkers progresses rapidly, with ongoing developments for both publicly reported biomarkers and biomarkers used in individual development. This article will discuss the current landscape in biomarker research and highlight strategies being adopted to increase the likelihood of transitioning biomarkers from discovery to medical practice to enable more objective decision making, and to improve health outcome. Translational research plays a key role in drug development and biomarker discovery for hepatocellular carcinoma (hcc). however, unique challenges exist in this field because of the limited availability of human tumor samples from surgery, the lack of homogenous oncogenic driver mutations, and the paucity of adequate experimental models. in this review, we provide insights into these. Biomarkers play important roles in the practice of medicine and all phases of drug development by providing information on patient status in the clinical setting and critical information on pharmacodynamic activity, efficacy, and safety throughout drug development. in some cases, biomarkers may even be used as surrogate endpoints for the basis.

Ibmcb Biomarker Discovery Translational Research Translational research plays a key role in drug development and biomarker discovery for hepatocellular carcinoma (hcc). however, unique challenges exist in this field because of the limited availability of human tumor samples from surgery, the lack of homogenous oncogenic driver mutations, and the paucity of adequate experimental models. in this review, we provide insights into these. Biomarkers play important roles in the practice of medicine and all phases of drug development by providing information on patient status in the clinical setting and critical information on pharmacodynamic activity, efficacy, and safety throughout drug development. in some cases, biomarkers may even be used as surrogate endpoints for the basis. In the perspective of drug development, biomarkers serve as an outstanding type of objective measuring parameters to facilitate whole cycle assessment of numerous bio pharmaceutical products, covering clinical pharmacology, companion diagnosis, safety risk prevention, among others [2], [6]. impressively in recently years, novel biomarkers have. Abstract. despite prodigious advances in tumor biology research, few tumor biomarker tests have been adopted as standard clinical practice. this lack of reliable tests stems from a vicious cycle of undervaluation, resulting from inconsistent regulatory standards and reimbursement, as well as insufficient investment in research and development.

Biomarker Discovery Proteomics For Translational Biomarker Research In the perspective of drug development, biomarkers serve as an outstanding type of objective measuring parameters to facilitate whole cycle assessment of numerous bio pharmaceutical products, covering clinical pharmacology, companion diagnosis, safety risk prevention, among others [2], [6]. impressively in recently years, novel biomarkers have. Abstract. despite prodigious advances in tumor biology research, few tumor biomarker tests have been adopted as standard clinical practice. this lack of reliable tests stems from a vicious cycle of undervaluation, resulting from inconsistent regulatory standards and reimbursement, as well as insufficient investment in research and development.

Comments are closed.